Uranyl Speciation in Carbonate-Rich Hydrothermal Solutions: A Molecular Dynamics Study
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
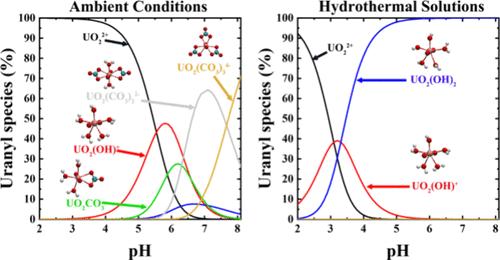
In this study, we employed classical molecular dynamics (CMD) and first-principles molecular dynamics (FPMD) simulations to investigate the speciation of uranyl in carbonate-rich hydrothermal solutions. The association constants (log10KA) of uranyl carbonate complexes were derived from the potential of mean forces (PMFs) obtained from CMD simulations, and the acid constants (pKas) of uranyl aqua ions were calculated using the FPMD-based vertical energy gap method. The results showed that uranyl ions could form stable mono- and bi-carbonate complexes at elevated temperatures and that uranyl aqua ions strongly hydrolyzed in neutral solutions at temperatures exceeding 473 K. The speciation of uranyl in hydrothermal solutions was constructed based on the calculated thermodynamics data. It was found that uranyl carbonate complexes were predominant in aqueous solutions at temperatures below 373 K, and at higher temperatures, UO2(OH)2/UO2(OH)+ became the predominant species. These findings provide molecular-level insight into the speciation of uranyl in hydrothermal solutions and highlight the role of uranyl hydroxides in the transport and deposition of uranium in hydrothermal processes.
富碳酸盐水热溶液中铀酰形态的分子动力学研究
本研究采用经典分子动力学(CMD)和第一性原理分子动力学(FPMD)模拟研究了铀酰在富碳酸盐水热溶液中的形态。利用CMD模拟得到的平均力势(PMFs)推导出了碳酸铀酰配合物的缔合常数log10KA,并利用基于fpmd的垂直能隙法计算出了碳酸铀酰水离子的酸常数pKas。结果表明,铀酰离子在高温下可形成稳定的单碳酸和双碳酸配合物,且铀酰水合离子在超过473 K的中性溶液中发生强水解。根据计算得到的热力学数据,建立了铀酰在水热溶液中的形态。在373 K以下的水溶液中以碳酸铀酰配合物为主,在较高的温度下以UO2(OH)2/UO2(OH)+为主。这些发现为热液溶液中铀酰的形态提供了分子水平的见解,并突出了铀酰氢氧化物在热液过程中铀的运输和沉积中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


