Bone-Marrow-Targeted Nanocomposite Abrogates C-Myb-Survivin Cross Talk in MLL-AF9-Rearranged Acute Myeloid Leukemia in In Vitro and In Vivo Patient-Derived Xenograft Models
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The heterogeneous form of malignancy in the myeloid lineage of normal hematopoietic stem cells (HSCs) is characterized as acute myeloid leukemia (AML). The t(9;11) reciprocal translocation (p22;q23) generates MLL-AF9 oncogene, which results in myeloid-based monoblastic AML with frequent relapse and poor survival. MLL-AF9 binds with the C-Myb promoter and regulates AML onset, maintenance, and survival. The bone marrow microenvironment (BMM) protects leukemia stem cells (LSCs) from therapeutic agents, which can lead to relapsed condition. Targeting leukemia BMM can be a viable therapeutics approach for AML treatment, wherein bone homing bisphosphonate, ibandronic acid (IBD), can localize to the BMM. In order to target the BMM of AML, C-Myb siRNA was entrapped in Vitamin D nanoemulsion-functionalized with BMM-targeted IBD, which exhibited binding with ex vivo bone slices and localization into mice bone marrow. IBD functionalization and C-Myb siRNA nanotherapy enhanced the suppression of LSCs (c-Kit+) and the upregulation of myeloid differentiation markers CD11b and Gr-1 in peripheral blood and bone marrow of athymic nude mice and patient-derived xenograft models. IBD functionalization enhanced the downregulation of C-Myb and C-Myb-Survivin cross talk in bone marrow and spleen tissue responsible for AML onset, maintenance, and pathogenesis. Further C-Myb binding to Survivin promoter was abrogated by the present bone-marrow-targeted nanotherapy, signifying its translational potential for AML therapeutics.
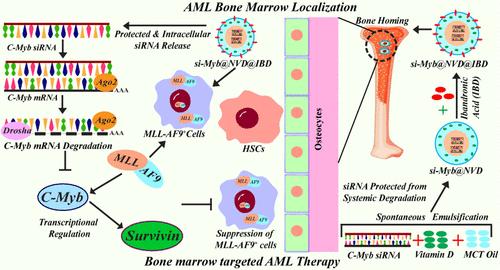
骨髓靶向纳米复合材料在体外和体内患者来源的异种移植模型中消除mll - af9重排急性髓系白血病中的C-Myb-Survivin串扰
在正常造血干细胞(hsc)的髓系系中,恶性肿瘤的异质形式被描述为急性髓系白血病(AML)。t(9;11)互易易位(p22;q23)产生mml - af9癌基因,导致以骨髓为基础的单核细胞AML复发频繁,生存率差。MLL-AF9与C-Myb启动子结合,调节AML的发病、维持和生存。骨髓微环境(BMM)保护白血病干细胞(LSCs)免受治疗药物的影响,治疗药物可导致病情复发。靶向白血病BMM可能是AML治疗的一种可行的治疗方法,其中骨归巢双膦酸,IBD,可以定位到BMM。为了靶向AML的BMM, C-Myb siRNA被包裹在BMM靶向IBD的维生素D纳米乳中,其与离体骨片结合并定位到小鼠骨髓中。IBD功能化和C-Myb siRNA纳米疗法增强了胸腺裸鼠和患者来源的异种移植模型外周血和骨髓中LSCs (c-Kit+)的抑制和骨髓分化标志物CD11b和Gr-1的上调。IBD功能化增强了骨髓和脾脏组织中与AML发病、维持和发病有关的C-Myb和C-Myb- survivin串音的下调。目前的骨髓靶向纳米疗法进一步消除了C-Myb与Survivin启动子的结合,这表明其在AML治疗中的转化潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


