Optimizing Oxygen Vacancies through p-Band Center Modulation of Oxygen in the Li2WO4/Mg6MnO8 Catalyst for Enhanced Oxidative Coupling of Methane: An Experimental and Theoretical Study
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
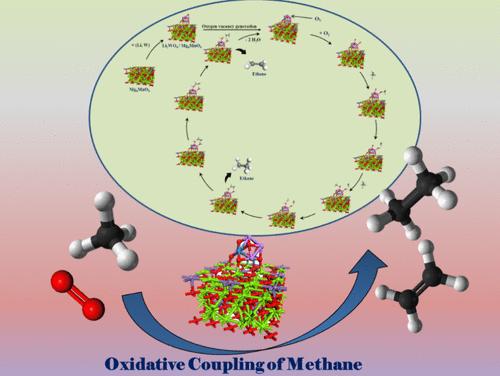
Herein, we demonstrate a one-pot sol–gel-assisted procedure to prepare a defect-rich Li2WO4/Mg6MnO8 catalyst having surface oxygen vacancies, which facilitates the adsorption of O2 molecules to generate active oxygen species (O2–, O22–) by incorporating Li and W into the Mg6MnO8 lattice. These active oxygen species serve as primary active sites, selectively dissociating CH4 into CH3• and promoting CH3• coupling into C2H6, while hindering excessive oxidation of CH3• into COx. Various analytical methods such as XPS, O2-TPD, EPR, CH4-TPSR, in situ DRIFTS, and in situ Raman spectroscopy studies demonstrated that surface reactive oxygen species are more active and selective than lattice oxygen toward the formation of C2 products. The controlled addition of Li and W plays a crucial role in stabilizing surface Li species through the formation of Li–O–W bonds by forming the Li2WO4 phase, ensuring stable catalyst performance up to 100 h. DOS analysis shows a positive shift in the p-band center, which effectively promotes the formation of oxygen vacancies. Analytical studies confirmed that surface active oxygen species are more active and selective than lattice oxygen in forming C2 hydrocarbons. The Li2WO4/Mg6MnO8 catalyst exhibited superior performance, achieving ∼82% C2 selectivity and ∼25% C2 yield at 700 °C. We found that the stable formation of active oxygen species (O2–) and a high Mn4+/Mn3+ ratio over the surface are the key factors for achieving high C2 selectivity and yield during OCM. DFT results show that the concentration of oxygen defect sites is higher on the surface of the Li2WO4/Mg6MnO8 catalyst, which synergistically binds Li2WO4 and Mg6MnO8, in comparison with pure Mg6MnO8 surfaces. Furthermore, DFT calculations also indicate that oxygen vacancies are energetically more favorable on the surface of the Li2WO4/Mg6MnO8 catalyst rather than in its subsurface. In situ XRD and in situ Raman analysis demonstrated that Li2WO4 undergoes a reversible phase change, transitioning into a molten state at higher temperatures, potentially forming Li2O2 species that may serve as active centers during the reaction.
通过p波段中心调制Li2WO4/Mg6MnO8催化剂中氧空位的优化以增强甲烷氧化偶联:实验和理论研究
在此,我们展示了一种一锅溶胶-凝胶辅助工艺,以制备具有表面氧空位的富缺陷Li2WO4/Mg6MnO8催化剂,通过将Li和W结合到Mg6MnO8晶格中,促进O2分子吸附生成活性氧(O2 -, O22 -)。这些活性氧作为主要活性位点,选择性地将CH4解离成CH3•,促进CH3•偶联成C2H6,同时阻止CH3•过度氧化成COx。各种分析方法,如XPS、O2-TPD、EPR、CH4-TPSR、原位漂移和原位拉曼光谱研究表明,表面活性氧比晶格氧对C2产物的形成更具活性和选择性。通过形成Li2WO4相形成Li - o - W键,Li和W的可控添加对稳定表面Li物种起着至关重要的作用,确保了催化剂长达100 h的稳定性能。DOS分析显示p带中心正偏移,这有效地促进了氧空位的形成。分析研究证实,表面活性氧在形成C2烃方面比晶格氧具有更强的活性和选择性。Li2WO4/Mg6MnO8催化剂表现出优异的性能,在700°C下实现了~ 82%的C2选择性和~ 25%的C2产率。我们发现,在OCM过程中,活性氧(O2 -)的稳定形成和表面Mn4+/Mn3+的高比率是实现高C2选择性和产率的关键因素。DFT结果表明,与纯Mg6MnO8表面相比,Li2WO4/Mg6MnO8催化剂表面的氧缺陷位点浓度更高,其协同结合Li2WO4和Mg6MnO8表面。此外,DFT计算还表明,氧空位在Li2WO4/Mg6MnO8催化剂的表面上比在其表面下更有利。原位XRD和原位拉曼分析表明,Li2WO4经历了可逆的相变,在较高的温度下转变为熔融状态,可能形成Li2O2物质,在反应过程中可能作为活性中心。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


