Cu-Catalyzed Diastereo- and Enantioselective Synthesis of Homopropargyl Amines Bearing All-Carbon Quaternary Stereocenters via Chirality Transfer of Hindered Allenylcopper Species
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The construction of congested acyclic stereocenters with high stereoselectivity is a significant challenge in synthetic chemistry. Herein, we report an efficient method for diastereo- and enantioselective C–C coupling of 1,3-disubstituted enynes with imines for the asymmetric construction of vicinal stereogenic centers, including an all-carbon quaternary center. This coupling was accomplished by chirality transfer from axial-to-central of fully substituted axially chiral allenylcopper intermediates formed in situ from branched enynes with concomitant diastereoselective formation of an additional stereocenter in imine addition enabled by a chiral C1-symmetric N-heterocyclic carbene (NHC) copper catalyst. DFT calculations provided an enhanced understanding of the silyl effect of allenylcopper nucleophiles on reactivity and the origin of stereoselectivity. Synthetic versatility of the resulting products bearing densely functionalized groups could amplify the significance of the current method.
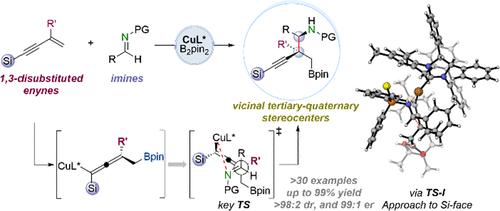
通过阻碍烯基铜的手性转移,铜催化非对映和对映选择性合成含全碳季立体中心的同丙基胺
构建具有高立体选择性的密集无环立体中心是合成化学中的一个重大挑战。在此,我们报道了一种有效的非映对和对映选择性的1,3-二取代炔与亚胺的C-C偶联方法,用于不对称构建邻立体中心,包括全碳季元中心。这种偶联是通过手性c1对称n -杂环碳烯(NHC)铜催化剂在亚胺加成过程中,由支链烯烃原位形成的完全取代的轴向手性烯基铜中间体的轴向中心手性转移而实现的,并伴有非对映选择性地形成一个额外的立体中心。DFT计算提高了对烯基铜亲核试剂对反应性的硅基效应和立体选择性的来源的理解。所得到的含有密集官能团的产物的合成通用性可以放大当前方法的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


