Modulating Lattice Oxygen through an Alkaline Earth Metal Promoter for Chemical Looping Oxidative Dehydrogenation of Propane
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Rational design of metal oxide-type redox catalysts for selective propylene production is of paramount importance, yet remains challenging. This paper describes the structural and electronic effect of an alkaline earth metal promoter on lattice oxygen reactivity for chemical looping oxidative dehydrogenation (CL-ODH) of propane through the configuration of core–shell-type redox catalysts, which consist of a redox-active FeVO4 core and a selective mixed alkaline earth metal oxide shell. A systematic study demonstrates that Mg is the optimal promoter among all alkali and alkaline earth metals investigated, and the formed Mg2V2O7 outer shell provides a catalytic surface for C–H activation while blocking the nonselective sites for FeVO4, typically as an oxygen carrier. The core–shell redox catalyst with a higher coverage of the Mg2V2O7 layer achieves an enhanced propylene selectivity of 80.8% at an operation temperature of 550 °C. The design strategy highlights the exploration of alkaline earth metals in redox catalysts for chemical looping processes.
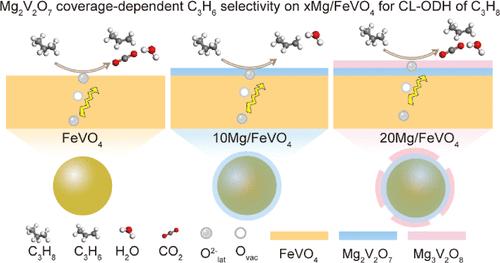
用碱土金属促进剂调制晶格氧在丙烷化学环氧化脱氢中的作用
合理设计金属氧化物型氧化还原催化剂用于选择性丙烯生产是至关重要的,但仍然具有挑战性。本文通过核-壳型氧化还原催化剂的配置,描述了碱土金属促进剂对丙烷化学环氧化脱氢(CL-ODH)晶格氧反应活性的结构和电子效应,该催化剂由氧化还原活性FeVO4核和选择性混合碱土金属氧化物壳组成。系统研究表明,Mg是所研究的碱和碱土金属中最理想的促进剂,形成的Mg2V2O7外壳为C-H活化提供了催化表面,同时阻断了FeVO4的非选择性位点,通常作为氧载体。在550℃的操作温度下,具有较高Mg2V2O7层覆盖率的核壳氧化还原催化剂的丙烯选择性提高了80.8%。该设计策略突出了碱土金属在化学环过程氧化还原催化剂中的探索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
文献相关原料
公司名称
产品信息
阿拉丁
Ba(NO3)2
阿拉丁
Sr(NO3)2
阿拉丁
Ca(NO3)2·4H2O
阿拉丁
Mg(NO3)2·6H2O
阿拉丁
KNO3
阿拉丁
NaNO3
阿拉丁
LiNO3
阿拉丁
NH4VO3
阿拉丁
Ba(NO3)2
阿拉丁
Sr(NO3)2
阿拉丁
Ca(NO3)2·4H2O
阿拉丁
Mg(NO3)2·6H2O
阿拉丁
KNO3
阿拉丁
NaNO3
阿拉丁
LiNO3
阿拉丁
NH4VO3
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


