Highly Stable Subnanometric Pt Clusters in All Silica K-Doped Zeolites: Implications for the CO Oxidation Reaction
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
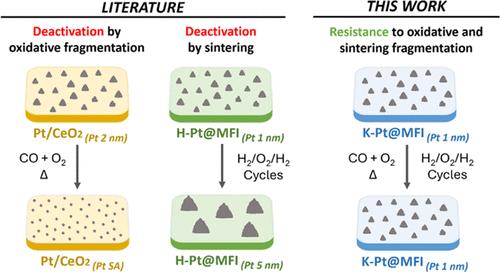
Small metal clusters can provide improved catalytic activity compared with single metal atoms and larger metal nanoparticles of the same element. The stabilization of metal ensembles of a few atoms is extremely challenging, however, because reductive sintering and oxidative fragmentation are phenomena that often occur at low temperatures in reactive atmospheres. In this regard, the CO oxidation reaction is particularly challenging because CO tends to aggregate noble metals on nonreducible supports, such as SiO2, whereas O2 triggers the formation of (less active) single atoms on reducible supports, such as CeO2. Accordingly, state-of-the-art Pt/CeO2 catalysts undergo severe deactivation under practical CO oxidation conditions in excess of O2. In this contribution, we report a highly active CO oxidation catalyst that is able to overcome both sintering and fragmentation instabilities under conditions that make other alternatives fail. The catalyst is based on small Pt clusters inside K-MFI that benefit from both strong metal/support interactions at defective sites of the zeolite and strong electronic promotion by the support, to attain highly stable, highly active, electron-rich Pt clusters.
高稳定的亚纳米Pt团簇在所有二氧化硅k掺杂沸石:对CO氧化反应的影响
与单个金属原子和相同元素的较大金属纳米颗粒相比,小金属团簇可以提供更好的催化活性。然而,由于还原性烧结和氧化碎裂是在低温反应气氛中经常发生的现象,少数原子金属系的稳定是极具挑战性的。在这方面,CO氧化反应尤其具有挑战性,因为CO倾向于在不可还原的载体(如SiO2)上聚集贵金属,而O2则会在可还原载体(如CeO2)上形成(活性较低的)单原子。因此,最先进的Pt/CeO2催化剂在实际CO氧化条件下,在超过O2的情况下会发生严重的失活。在这篇文章中,我们报道了一种高活性的CO氧化催化剂,它能够克服烧结和破碎不稳定的条件,使其他替代品失败。该催化剂基于K-MFI内部的小Pt团簇,这些小Pt团簇受益于沸石缺陷部位的强金属/载体相互作用和载体的强电子促进,从而获得高度稳定、高活性、富电子的Pt团簇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


