Nitrogen-doped porous hydrochar for enhanced chromium(VI) and bisphenol A scavenging: Synergistic effect of chemical activation and hydrothermal doping
IF 7.7
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
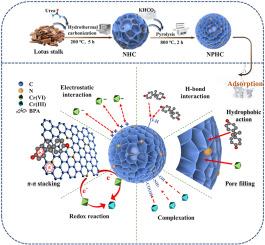
Nitrogen-doped porous hydrochar (NPHC) was successfully synthesized by hydrothermal carbonization and activation with KHCO3, which was employed for scavenging hexavalent chromium (Cr(VI)) and bisphenol A (BPA) in contaminated water. N doping increased the unique active sites such as amino and molecular N in NPHC for adsorbing contaminants, and enhanced the activation effect. Compared to original (HC) and N-doped hydrochar (NHC), the SBET of material improved from 3.99 m2/g and 4.71 m2/g to 1176.77 m2/g. Meanwhile, NPHC exhibited more superior adsorption capacity for Cr(VI) (323.25 mg/g) and BPA (545.34 mg/g) than that of porous hydrochar (213.17 and 343.67 mg/g). Moreover, NPHC possessed pH-dependence and presented more excellent tolerance for interfering ions and regeneration performance. Notably, the Cr(VI) capture by NPHC was dominated via pore filling, electrostatic interaction, reduction, and complexation, while π-π stacking, H-bond interaction, and hydrophobic action were relevant to the binding mechanism of BPA. Overall, the proposed functionalization strategy for biochar was conducive to enhance the remediation of water bodies.
氮掺杂多孔炭增强铬(VI)和双酚A的清除作用:化学活化和水热掺杂的协同效应。
通过水热炭化和KHCO3活化制备了氮掺杂多孔炭(NPHC),并将其用于去除污染水中的六价铬(Cr(VI))和双酚A (BPA)。N掺杂增加了NPHC吸附污染物的氨基酸和分子N等独特活性位点,增强了活化效果。与原始(HC)和n掺杂氢炭(NHC)相比,材料的SBET从3.99 m2/g和4.71 m2/g提高到1176.77 m2/g。同时,NPHC对Cr(VI) (323.25 mg/g)和BPA (545.34 mg/g)的吸附能力优于多孔炭(213.17 mg/g和343.67 mg/g)。NPHC具有ph依赖性,对干扰离子的耐受性和再生性能更优异。值得注意的是,NPHC捕获Cr(VI)主要通过孔隙填充、静电相互作用、还原和络合作用,而π-π堆积、氢键相互作用和疏水作用与BPA的结合机制有关。综上所述,生物炭功能化策略有利于增强水体的修复能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Research
环境科学-公共卫生、环境卫生与职业卫生
CiteScore
12.60
自引率
8.40%
发文量
2480
审稿时长
4.7 months
期刊介绍:
The Environmental Research journal presents a broad range of interdisciplinary research, focused on addressing worldwide environmental concerns and featuring innovative findings. Our publication strives to explore relevant anthropogenic issues across various environmental sectors, showcasing practical applications in real-life settings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


