Tuning the electron structure of single-atom Fe catalyst for designed peroxymonosulfate activation and phenols degradation
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
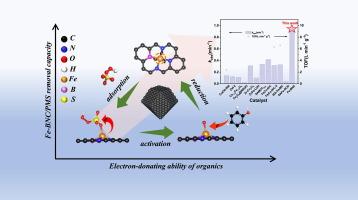
Transition metal single atom catalysts (SACs) for boosting peroxymonosulfate (PMS) activation involving complex catalytic mechanism and multiple reaction pathways have received much attention, but regulating the reaction pathway of SACs is still an important challenge in the PMS-mediated Fenton-like reaction. Herein, a boron-doped Fe SAC with FeN3B configurations (Fe—BNC) was synthesized and selectively generated a non-radical pathway, in which the high-valent iron-oxo species (FeIV![]() O) were determined as the main reactive oxygen species (ROS) by PMS activation. The Fe-BNC/PMS system not only exhibited remarkable reaction kinetic constant (0.949 min−1) and turnover frequency (9.49 L min−1g−1) for the phenol degradation, but also showed excellent selectivity to phenols with strong electron-donating ability. Mechanism exploration based on theoretical calculations revealed that high activity of Fe-BNC originated from the reinforced adsorption energy and enhanced overlap between Fe 3d and O 2p orbits, which facilitated to strengthen the Fe-O bonding and accelerate the electron transfer, thus modulating the PMS activation via a non-radical pathway. Successful extendibility of Fe-BNC in treatment of the real water and coking wastewater demonstrates its application potential. This work elucidates the mechanism of selectively generating a non-radical pathway over B-doped Fe-based SAC, and provides a rational strategy for preparing SACs alone with a non-radical pathway.
O) were determined as the main reactive oxygen species (ROS) by PMS activation. The Fe-BNC/PMS system not only exhibited remarkable reaction kinetic constant (0.949 min−1) and turnover frequency (9.49 L min−1g−1) for the phenol degradation, but also showed excellent selectivity to phenols with strong electron-donating ability. Mechanism exploration based on theoretical calculations revealed that high activity of Fe-BNC originated from the reinforced adsorption energy and enhanced overlap between Fe 3d and O 2p orbits, which facilitated to strengthen the Fe-O bonding and accelerate the electron transfer, thus modulating the PMS activation via a non-radical pathway. Successful extendibility of Fe-BNC in treatment of the real water and coking wastewater demonstrates its application potential. This work elucidates the mechanism of selectively generating a non-radical pathway over B-doped Fe-based SAC, and provides a rational strategy for preparing SACs alone with a non-radical pathway.

调整单原子铁催化剂的电子结构,用于设计过氧单硫酸盐活化和酚类降解
过渡金属单原子催化剂(SAC)用于促进过一硫酸盐(PMS)活化,涉及复杂的催化机理和多种反应途径,已受到广泛关注,但调节SAC的反应途径仍是PMS介导的类芬顿反应中的一个重要挑战。本文合成了一种具有 FeN3B 构型的掺硼铁 SAC(Fe-BNC),并选择性地产生了一种非自由基途径,其中高价铁氧物种(FeIVO)被确定为 PMS 活化的主要活性氧物种(ROS)。FeBNC/PMS 系统不仅在苯酚降解方面表现出显著的反应动力学常数(0.949 min-1)和周转频率(9.49 L min-1g-1),而且对电子供能能力强的苯酚表现出极佳的选择性。基于理论计算的机理探索表明,FeBNC 的高活性源于吸附能的增强和 Fe 3d 与 O 2p 轨道重叠的增强,这有利于加强 FeO 键合和加速电子转移,从而通过非自由基途径调节 PMS 的活化。FeBNC 在实际水和焦化废水处理中的成功推广证明了其应用潜力。这项研究阐明了掺杂 B 的铁基 SAC 选择性产生非辐射途径的机理,并为制备单独具有非辐射途径的 SAC 提供了合理的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
文献相关原料
公司名称
产品信息
麦克林
Methyl phenyl sulfoxide
麦克林
5,5-dimethyl-1-pyrrolidine-N-oxide
麦克林
Furfuryl alcohol
麦克林
Sodium thiocyanate
麦克林
tert-butanol
麦克林
p-nitrophenol
麦克林
4-methoxyphenol
麦克林
Acetaminophen
麦克林
o-nitrophenol
麦克林
2,4-dichlorophenol
麦克林
Bisphenol A
麦克林
4-chlorophenol
麦克林
Phenol
麦克林
Boric acid
麦克林
2-methylimidazole
麦克林
Ferrous chloride tetrahydrate
麦克林
Zinc acetate dihydrate
阿拉丁
2,2,6,6-tetramethylpiperidine
阿拉丁
Chloroform
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


