Biomimetic “Trojan Horse” Fibers Modulate Innate Immunity Cascades for Nerve Regeneration
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
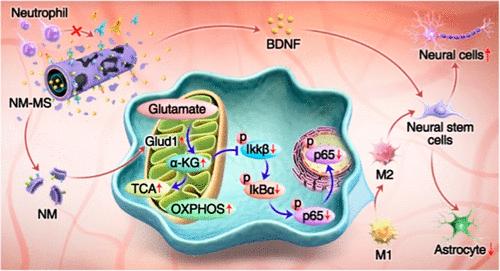
Neutrophil membrane vesicles (NMVs) have been successfully applied to control the inflammatory cascade after spinal cord injury (SCI) by acting as an inflammatory factor decoy to front-load the overall inflammation regulatory window; however, the mechanisms by which NMVs regulate macrophage phenotypic shifts as well as their outcomes have rarely been reported. In this study, we demonstrated the “efferocytosis-like” effect of NMVs endocytosed by macrophages, supplementing the TCA cycle intermediate metabolite α-KG by promoting glutamine metabolism, which in turn facilitates oxidative phosphorylation and inhibits the NF-κB signaling pathway to reprogram inflammatory macrophages to the pro-regenerative phenotype. Based on these findings, a “Trojan horse” composite fiber scaffold was constructed; this comprised a carboxylated poly-l-lactic acid shell encapsulated with NMVs and a core loaded with brain-derived neurotrophic factor to spatiotemporally modulate the inflammatory microenvironment by 39.23% and sustainably promote nerve regeneration by 85.67%. In vivo experiments further confirmed the effect of NMV-coated fiber scaffolds on the regulation of early innate immune inflammation and the continuous promotion of nerve regeneration. This study not only further unravels the mechanism of neutrophil membrane–macrophage interactions but also provides a strategy for coordinating inflammatory reprogramming and nerve regeneration following SCI.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


