Spatiotemporal metabolic mapping of ex-situ preserved hearts subjected to dialysis by integration of bio-SPME sampling with non-targeted metabolipidomic profiling
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
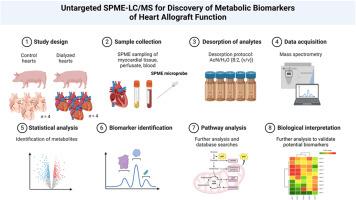
Normothermic ex situ heart perfusion (ESHP) has emerged as a valid modality for advanced cardiac allograft preservation and conditioning prior to transplantation though myocardial function declines gradually during ESHP thus limiting its potential for expanding the donor pool. Recently, the utilization of dialysis has been shown to preserve myocardial and coronary vasomotor function. Herein, we sought to determine the changes in myocardial metabolism that could support this improvement.Results
Male Yorkshire porcine hearts were subjected to ESHP for 8 hours with or without dialysis. Alterations in metabolism were studied with an innovative in vivo solid-phase microextraction (SPME) technology coupled with global metabolite profiling at 15 min, 1.5, 4, and 8 hours of perfusion. Bio-SPME sampling was performed by inserting SPME fibres coated with a PAN-based extraction phase containing mixed-mode (C8+benzenesulfonic acid) functionalities into the myocardium to a depth of their entire 8 mm coating or immersing them in the perfusate, followed by a 20-minute extraction period for the analytes of interest. Dialyzed hearts demonstrated improved bioenergetics as evidenced by accelerated purine metabolism and less pronounced accumulation of intermediates of fatty acid β/ω-oxidation. Metabolic waste accumulation such as pro-inflammatory lipid mediators (e.g., leukotrienes) was mitigated thereby supporting the process of resolution of inflammation through excretion of specialized pro-resolving mediators (resolvins D1/D2, E2, protecin D1).Significance
Through implementing the unique analytical pipeline we demonstrated that the addition of dialysis may preserve cardiac metabolism allowing for prolonged ESHP. This strategy has the potential to facilitate high-risk donor organs’ reconditioning prior to transplantation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


