Naphthalene Hydrodearomatization via Controllable Photocatalytic Hydroboration
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
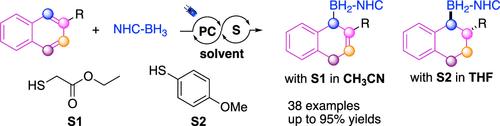
The photocatalytic dearomative 1,4-hydroboration of naphthalenes with an N-heterocyclic carbene borane (NHC-BH3) complex was reported herein with controllable regioselectivity and chemoselectivity. This protocol yielded a wide range of naphthalene derivatives bearing various functional groups, notably bioactive compounds. Hydroboration occurred through the cooperation of photoredox and hydrogen atom transfer via boryl radical addition to naphthalene and further selective protonation. Notably, tuning the conditions could lead to further hydrogenation of the tetrahydroboration products. Mechanistic studies, including experimental and computational studies, elucidated the mechanism for the regioselectivity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


