Binary solvents of anhydrous ethanol and propylene carbonate for electrolytes of amorphous WO3 films to improve electrochromic performance
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
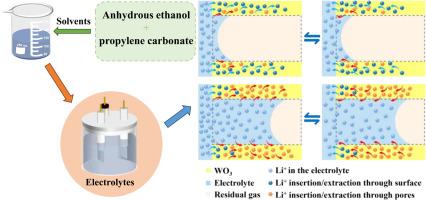
In this study, amorphous WO3 thin films were deposited on indium-tin oxide substrates using radio frequency magnetron sputtering. Anhydrous ethanol (AE) and propylene carbonate (PC) served as binary solvents, with the WO3 films evaluated in AE+PC-LiClO4 electrolytes. By adjusting the volume percentage of AE, the physical properties of the electrolytes were modified to improve the fluidity and wetting behavior. The analysis of several aspects, such as migration of ions, capillary flow of electrolyte and electrochemical reaction rate, highlights the important influence of AE+PC binary solvent in reducing the response time. Notably, WO3 films in AE+PC-LiClO4 electrolyte with a 7/8 volume percentage of AE demonstrated exceptional optical modulation (ΔT=75.7%) and fast response times (tc=6.41 s, tb=2.29 s), alongside shortened the diffusion path, increased ion diffusion coefficients (Da=4.4678 × 10−10 cm2/s, Dc =8.2968 × 10−10 cm2/s) and electrochemical active area. Based on these efforts, the feasibility of applying AE+PC binary solvent to WO3 electrochromic thin films has been confirmed. These results offer valuable insights for the development of efficient electrolytes and their application in electrochromic devices.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


