Developing Dual-Responsive Quinolinium Prodrugs of 8-Hydroxyquinoline By Harnessing the Dual Chelating Sites
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
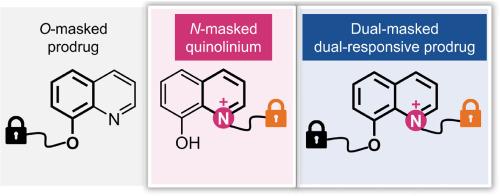
The bidentate metal ion chelator 8-hydroxyquinoline (8-HQ) demonstrates significant potential in anticancer therapy but is hindered by adverse effects due to nonspecific chelation in normal tissues. The phenolic hydroxyl oxygen of 8-HQ has been extensively exploited to develop O-masked 8-HQ prodrugs aimed at achieving on-demand chelation. However, the equally crucial quinoline nitrogen for chelation remains underutilized. By alkylating the quinoline nitrogen of 8-HQ, we synthesized a series of N-masked quinolinium (QUM) prodrugs that release 8-HQ upon activation by various stimuli. Comprehensive in vitro and in vivo studies were conducted with QUM-1 and QUM-4, which are activated by H2O2 and β-glucosidase, respectively. Both QUM-1 and QUM-4 exhibit improved cancer cell selectivity compared to 8-HQ or the O-masked isomeric prodrug, attributed to unique properties such as enhanced mitochondrial targeting and increased glucose transporter-mediated cellular uptake. Additionally, by leveraging both chelating sites, we constructed dual-masked 8-HQ prodrugs that are activated non-sequentially by two stimuli to release 8-HQ. QUM-5 demonstrates anticancer activity upon activation by UV/H2O2 and shows improved safety in mice compared to 8-HQ. Our research presents novel applications for the construction of quaternary ammonium prodrugs utilizing aromatic tertiary amines and underscores the potential of dual-responsive prochelators for targeted cancer therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


