Highly Conductive Non-Calcined 2D Cu0.3Co0.7 Bimetallic–Organic Framework for Urea Electrolysis in Simulated Seawater
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
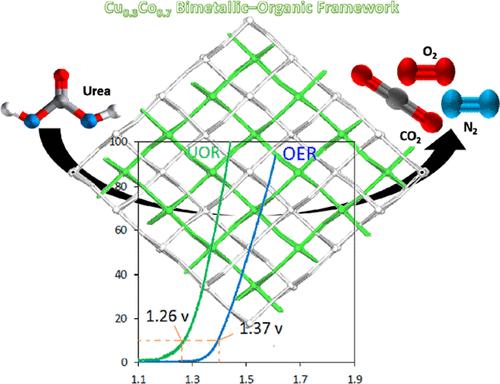
Global clean energy demands can be effectively addressed using the promising approach of hydrogen energy generation combined with less energy consumption. Hydrogen can be generated, and urea-rich wastewater pollution can be mitigated in a low-energy manner using the urea oxidation reaction (UOR). This paper seeks to assemble a unique electrocatalyst of a pristine 2D MOF, [Co(HBTC)(DMF)]n (Co-MUM-3), from 1,3,5-benzenetricarboxylate (BTC) to oxidize urea in simulated seawater. Ni foam (NF)-based working electrodes were fabricated by incorporating a series of heterometallic CuCo-MUM-3 frameworks (Cu0.1Co0.9-MUM-3, Cu0.2Co0.8-MUM-3, Cu0.3Co0.7-MUM-3, and Cu0.4Co0.6-MUM-3), after which their application in the urea oxidation reaction was examined. A very low required overpotential [1.26 V vs reversible hydrogen electrode (RHE) in 1 M KOH + 0.5 M NaCl (simulated seawater) + 0.33 M urea] and a Tafel slope of 112 mV dec–1 could be observed for the Cu0.3Co0.7-MUM-3 electrocatalyst, ensuring the achievement of urea electro-oxidation and hydrogen evolution reactions at a corresponding 10 mA cm–2 electrocatalytic current density. A relatively lower overpotential will be evident compared to other reported pristine MOFs, outperforming the commercial catalyst RuO2 (1.41 V at 10 mA cm–2, 131 mV dec–1) and ensuring considerable stability at significantly high current densities for a minimum of 72 h.
高导电性非煅烧二维Cu0.3Co0.7双金属-有机框架在模拟海水中尿素电解
利用氢能发电和减少能源消耗这一有前途的方法,可以有效地解决全球清洁能源需求。利用尿素氧化反应(UOR)可以产生氢气,以低能耗的方式减轻富尿素废水的污染。本文试图从1,3,5-苯三羧酸盐(BTC)中组装一种独特的二维MOF电催化剂[Co(HBTC)(DMF)]n (Co- mom -3),用于在模拟海水中氧化尿素。采用Cu0.1Co0.9-MUM-3、Cu0.2Co0.8-MUM-3、Cu0.3Co0.7-MUM-3、Cu0.4Co0.6-MUM-3等异质金属骨架制备了Ni泡沫(NF)基工作电极,并对其在尿素氧化反应中的应用进行了研究。在1 M KOH + 0.5 M NaCl(模拟海水)+ 0.33 M尿素条件下,cu0.3 co0.7 - mu -3电催化剂所需过电位为1.26 V vs可逆氢电极(RHE), Tafel斜率为112 mV dec1,确保在相应的10 mA cm-2电催化电流密度下实现尿素电氧化和析氢反应。与其他报道的原始mof相比,其过电位相对较低,优于商用催化剂RuO2 (10ma cm-2时1.41 V, 131mv dec1),并确保在高电流密度下至少72小时的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


