How reference electrodes improve our understanding of degradation processes in half and full cell potassium-ion battery setups
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
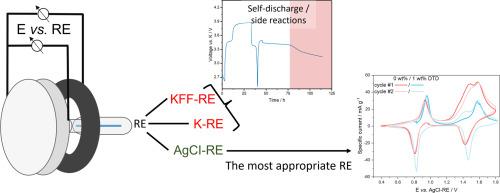
Electrochemical testing of electrodes for K-ion batteries (KIBs) can be strongly affected by interferences from electrolyte degradation reactions and associated crosstalk. This affects half cell measurements in particular. To address this issue, inert and stable reference electrodes are required for reliable 3-electrode measurements that allow to distinguish reversible electrochemical electrode processes more clearly from irreversible parasitic reactions. Therefore, this study evaluated K-metal and a partly charged positive electrode (K2Fe[Fe(CN)6], KFF), as two established solutions from the Li-ion battery field. Their electrochemical stability and suitability in various 3-electrode cell setups are evaluated in half and full cell, as well as symmetric cell configurations. Our experiments revealed that the high reactivity of the K-metal as reference or counter electrode interferes considerably with the electrode processes, leading to additional features in the voltage profile of KFF. Furthermore, any amount of K-metal led to a crosstalk-induced self-discharge of KFF. This places considerable limitations on the materials that can be used as a reference electrode. Therefore, we introduced an alternative reference electrode based on Ag/AgCl in a separate cell compartment, with high flexibility in the choice of the electrolyte formulation. To demonstrate the efficacy of this approach, we examine the impact of the electrolyte additive 1,3,2-dioxathiolane 2,2-dioxide (DTD) in 3-electrode setups, with the aim to illustrate the influence of DTD on the electrochemical processes of K-metal, KFF, and graphite electrodes in different cell configurations.

参考电极如何提高我们对半电池和全电池钾离子电池的降解过程的理解
电解液降解反应和相关串扰对k离子电池电极的电化学测试有很大的影响。这尤其影响半电池的测量。为了解决这个问题,需要惰性和稳定的参考电极进行可靠的3电极测量,以便更清楚地区分可逆电化学电极过程和不可逆寄生反应。因此,本研究评估了k金属和部分带电的正极(K2Fe[Fe(CN)6], KFF)作为锂离子电池领域的两种既定解决方案。他们的电化学稳定性和适用性在各种3电极电池设置在半和全电池,以及对称电池配置进行了评估。我们的实验表明,作为参比电极或对电极的k金属的高反应性极大地干扰了电极过程,导致KFF电压分布的额外特征。此外,任何量的k金属都可以引起KFF的串扰自放电。这对可用作参比电极的材料造成了相当大的限制。因此,我们在单独的电池室中引入了一种基于Ag/AgCl的替代参比电极,在选择电解质配方方面具有很高的灵活性。为了证明这种方法的有效性,我们研究了电解质添加剂1,3,2-二氧二硫代烷(DTD)在3电极设置中的影响,目的是说明DTD对k -金属、KFF和石墨电极在不同电池配置下的电化学过程的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


