Dynamic Stabilization of Cuδ+ in Heterostructured Ag0-CuAgOx for High-Performing Nitrate Electroreduction
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
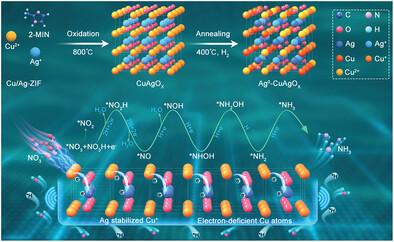
Oxide-derived copper (OD-Cu) has exhibited significant promise in nitrate electroreduction reaction (NO3RR) due to their hybrid Cu oxide states (Cuδ+) for stabilizing key reaction intermediates. However, owing to the intrinsic vulnerability of Cuδ+ reduction during NO3RR, it is still challenging to develop highly active and durable OD-Cu catalysts. Herein, a unique strategy is reported to stabilize the Cu+ state by dynamically introducing metallic Ag clusters in the oxidized CuAgOx nanosheets to form heterostructure Ag0-CuAgOx. Operando X-ray absorption spectroscopy and diffuse reflection infrared Fourier transform spectroscopy reveal a strong correlation between NH3 production and Cuδ+ content in Ag0-CuAgOx, with peak performance achieved when Cu+ is maximized. Ag0 acts as an electron acceptor, preventing the over-reduction of Cuδ+ during NO3RR. The stabilized Cu+ in Ag0-CuAgOx helps achieve outstanding long-term stability of 400 h for NH3 production, surpassing most of the state-of-the-art Cu-based electrocatalysts. Computational studies and ultraviolet photoelectron spectrometer confirm that Ag0 functions as the electronic buffer and enables electron transfer from Cu2O to Ag to generate electron-deficient Cu sites, thus turning the Cu d-band center with favorable adsorption energies for key intermediates to facilitate NH3 formation. The study paves the way to develop valence-stabilized catalysts for a range of electroreduction reactions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


