Cancer mutations rewire the RNA methylation specificity of METTL3-METTL14
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
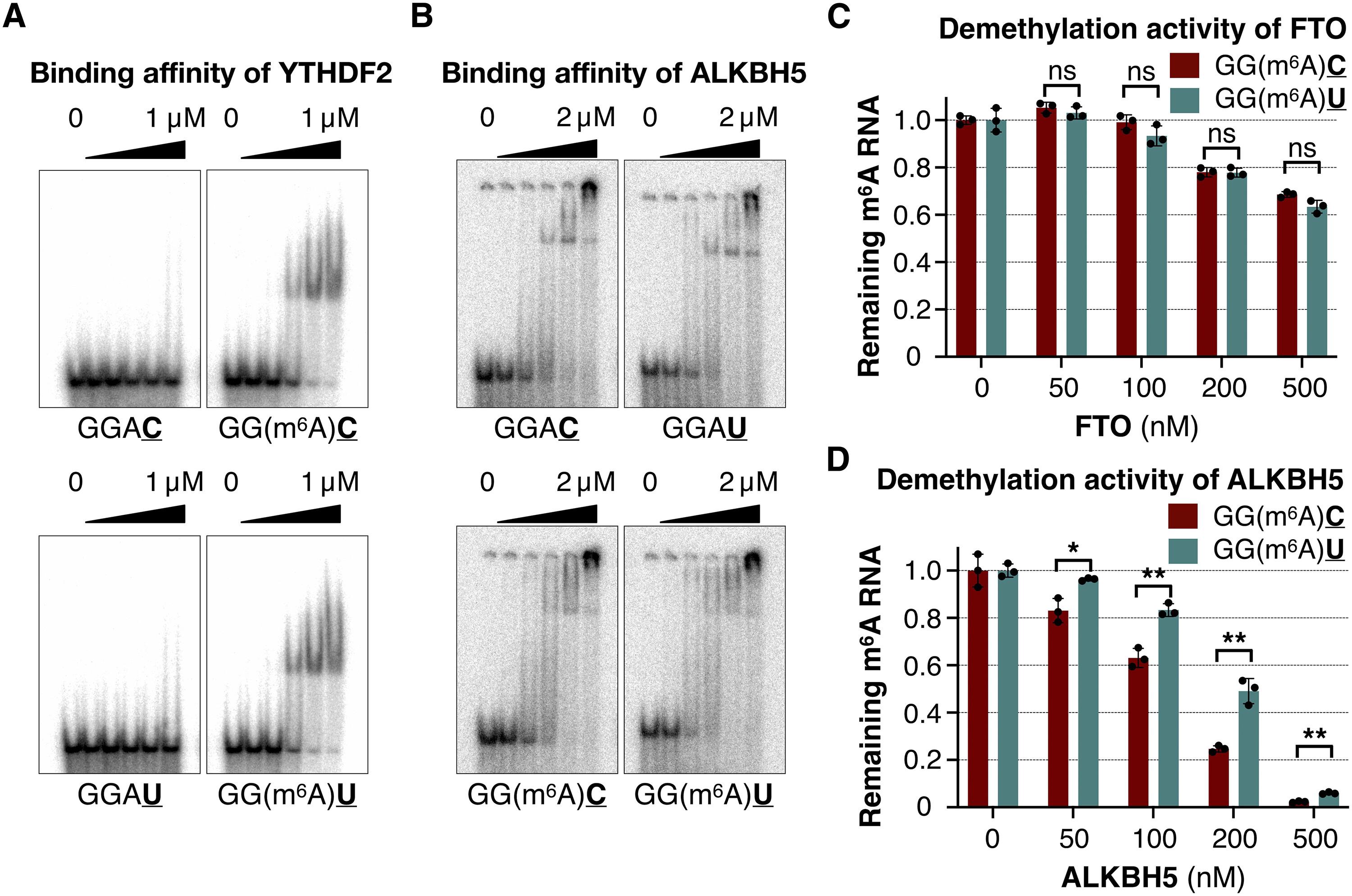
Chemical modification of RNAs is important for posttranscriptional gene regulation. The METTL3-METTL14 complex generates most N6-methyladenosine (m6A) modifications in messenger RNAs (mRNAs), and dysregulated methyltransferase expression has been linked to cancers. Here we show that a changed sequence context for m6A can promote oncogenesis. A gain-of-function missense mutation from patients with cancer, METTL14R298P, increases malignant cell growth in culture and transgenic mice without increasing global m6A levels in mRNAs. The mutant methyltransferase preferentially modifies noncanonical sites containing a GGAU motif, in vitro and in vivo. The m6A in GGAU context is detected by the YTH family of readers similarly to the canonical sites but is demethylated less efficiently by an eraser, ALKBH5. Combining the biochemical and structural data, we provide a model for how the cognate RNA sequences are selected for methylation by METTL3-METTL14. Our work highlights that sequence-specific m6A deposition is important and that increased GGAU methylation can promote oncogenesis.
癌症突变重新连接了METTL3-METTL14的RNA甲基化特异性
rna的化学修饰对转录后基因调控具有重要意义。METTL3-METTL14复合物在信使rna (mrna)中产生大多数n6 -甲基腺苷(m6a)修饰,甲基转移酶表达失调与癌症有关。在这里,我们表明改变m6a的序列背景可以促进肿瘤的发生。来自癌症患者的功能获得错义突变METTL14 R298P在培养和转基因小鼠中增加了恶性细胞的生长,但不增加mrna的全球mrna水平。在体外和体内,突变甲基转移酶优先修饰含有GGAU基序的非规范位点。与规范位点类似,GGAU背景下的m6a被YTH家族读取器检测到,但被一种擦除剂ALKBH5去甲基化的效率较低。结合生物化学和结构数据,我们为同源RNA序列如何被METTL3-METTL14选择甲基化提供了一个模型。我们的工作强调了序列特异性的m6a沉积是重要的,增加的GGAU甲基化可以促进肿瘤的发生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


