Mechanistic insights into carbonate radical-driven reactions: Selectivity and the hydrogen atom abstraction route
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
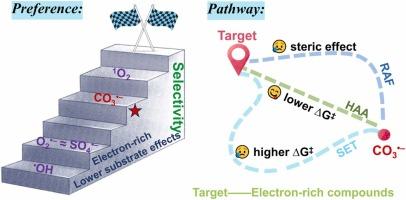
Carbonate radical (CO3• ) is inevitably produced in advanced oxidation processes (AOPs) when addressing real-world aqueous environments, yet it often goes unnoticed due to its relatively lower reactivity. In this study, we emphasized the pivotal role of CO3•
) is inevitably produced in advanced oxidation processes (AOPs) when addressing real-world aqueous environments, yet it often goes unnoticed due to its relatively lower reactivity. In this study, we emphasized the pivotal role of CO3• in targeting the elimination of contaminants by contrasting it with conventional reactive oxygen species (ROSs) and assessing the removal of sulfamethazine (SMT). Similar to singlet oxygen (1O2), CO3•
in targeting the elimination of contaminants by contrasting it with conventional reactive oxygen species (ROSs) and assessing the removal of sulfamethazine (SMT). Similar to singlet oxygen (1O2), CO3• shows a preference for electron-rich organic compounds. In addition, hydrogen atom abstraction (HAA) was determined as the primary pathway in CO3•
shows a preference for electron-rich organic compounds. In addition, hydrogen atom abstraction (HAA) was determined as the primary pathway in CO3• -driven reactions, with a lower free energy barrier (∆G‡) compared to the addition process, while single electron transfer (SET) was found to be thermodynamically unfavorable in all selected aromatics with varying substituents, using DFT calculations. The H atoms within amino groups (
-driven reactions, with a lower free energy barrier (∆G‡) compared to the addition process, while single electron transfer (SET) was found to be thermodynamically unfavorable in all selected aromatics with varying substituents, using DFT calculations. The H atoms within amino groups ( NH2 and
NH2 and  NH
NH ) were shown to be the most susceptible to abstraction by CO3•
) were shown to be the most susceptible to abstraction by CO3• , which is more facile than hydroxyl radical (•OH) due to the shorter N
, which is more facile than hydroxyl radical (•OH) due to the shorter N H bond cleavage length. Finally, the degradation intermediates of SMT by CO3•
H bond cleavage length. Finally, the degradation intermediates of SMT by CO3• were identified, with SO2 extraction, the cleavage of S
were identified, with SO2 extraction, the cleavage of S N and C
N and C N bonds, and nitration/nitrosation of
N bonds, and nitration/nitrosation of  NH2 groups being the main degradation pathways. The results from this study are expected to set the stage for the large-scale utilization of CO3•
NH2 groups being the main degradation pathways. The results from this study are expected to set the stage for the large-scale utilization of CO3• and advance our understanding of its reaction characteristics.
and advance our understanding of its reaction characteristics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


