Effect of Bisphenol F on Reproductive Function in F1 Generation Male Mice and its Potential Mechanisms
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
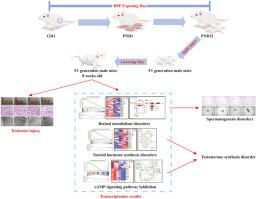
Bisphenol F (BPF) is an environmental endocrine disruptor capable of crossing the placental barrier and affecting the growth and development of offspring. Despite its potential impact, systematic research about effects of BPF on the reproductive function of male offspring remains limited. In this study, pregnant female mice were exposed to BPF at doses of 40, 400, and 4000 μg/kg during gestation and lactation, respectively, to evaluate its impact on testicular damage, testosterone levels, and spermatogenesis of male offspring (F1 generation), and further explore the mechanisms using transcriptomics. First, the study demonstrated that BPF induces testicular damage in F1 generation mice, leading to decreased testosterone levels and sperm quality. Second, transcriptomic analysis revealed that BPF affected spermatogenesis in F1 generation mice by disrupting retinol metabolism. Third, transcriptomic analysis revealed that BPF reduce the capacity for testosterone synthesis in F1 generation mice by diverting the testosterone precursor dehydroepiandrosterone (DHEA) towards the synthesis of 16α-hydroxydehydroepiandrosterone rather than testosterone. Finally, it was confirmed that BPF hinder cholesterol transport to mitochondria by inhibiting the cAMP signaling pathway, thereby impacting testosterone synthesis. In summary, the results of this study suggest that gestational exposure to BPF can lead to reproductive dysfunction in F1 generation male mice.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


