Pincer-Ruthenium-Catalyzed Direct Formation of Fuel-Grade Alkanes via a Net-Decarboxylative Coupling of Alcohols
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
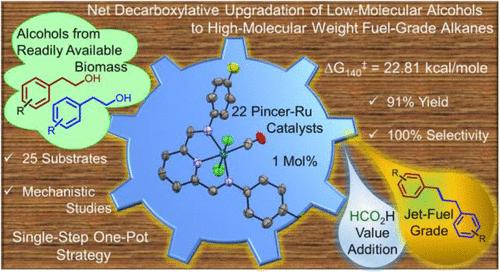
The net-decarboxylative coupling of low-molecular weight alcohols to high-molecular weight alkanes has been investigated using a series of NNN pincer-Ru catalysts based on bis(imino)pyridine and 2,6-bis(benzimidazole-2-yl)pyridine ligands. Notably, a majority of the considered pincer-Ru complexes, including the Ru precursors, were either not very active or were unselective giving alkene/alkane mixtures. However, in the presence of 0.5 equiv of NaOH in toluene at 140 °C, the complex (MeBim2NNN)RuCl2(PPh3)2 based on the 2,6-bis(benzimidazole-2-yl)pyridine ligand demonstrated very high activity giving up to 91% yield with 100% selectivity toward the alkane (1,3-diphenyl propane) starting from 2-phenyl ethanol after 24 h of reaction. On the other hand, the complex (iPr2NNN)RuCl2(PPh3) based on the bis(imino)pyridine ligand was found to be the least active and gave 14% 1,3-diphenyl propane at 25% selectivity. Experimental mechanistic studies point to the evolution of hydrogen (detected by GC) and formic acid (detected by 1H NMR) during the reaction along with the involvement of organic intermediates such as α,β-unsaturated aldehydes. The [(MeBim2NNN)RuCl(PPh3)2]Cl catalyzed transformation of 2-phenyl ethanol to 1,3-diphenyl propane demonstrated a first-order dependence of the initial rate on the concentration of both the catalyst and the base. While catalyst poisoning experiments with Hg revealed the homogeneous and well-defined molecular nature of the catalyst, a few of these molecular species, including the resting state (MeBim2NNN)RuHCl (experimentally trapped as its PPh3 adduct), have been identified by HRMS analysis and NMR studies. DFT studies complement the experimental findings and indicate that in the more favorable hydrogenolysis path, the dehydrogenolysis step is rate-determining (ΔG140‡ = 22.81 kcal/mol), and it leads to the formation of 2-phenyl acetaldehyde along with the resting state (MeBim2NNN)RuHCl. On the other hand, the corresponding cycle with the least active catalyst (iPr2NNN)RuCl2(PPh3) that involved the insertion of 1,3-diphenyl propene into the Ru–H bond as the RDS had a relatively more unfavorable barrier of 27.81 kcal/mol. This work that provides direct access to jet-fuel-grade 1,3-diphenyl propane starting from 2-phenyl ethanol in a single-step, one-pot strategy offers great promise to open up exciting opportunities in this very important field of study.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


