Syringable Microcapsules for Sustained, Localized, and Controllable siRNA Delivery
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
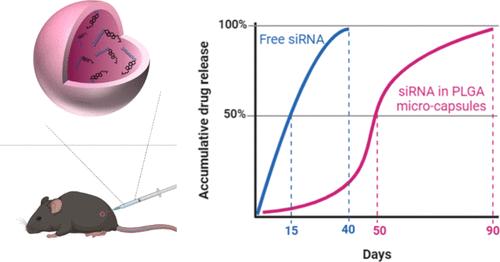
The clinical use of small interfering RNA (siRNA) and antisense oligonucleotides often requires invasive routes of administration, including intrathecal or intraocular injection. Additionally, these treatments often necessitate repeated injections. While nanoparticle formulation and chemical modifications have extended siRNA therapeutic durability, challenges persist, such as the side effects of bolus injections with high toxicity and maximum exposure in the acute phase. We present a microcapsule-based method to extend the activity of cholesterol-conjugated siRNA locally. Using microfluidics, microcapsules with well-defined size distribution and shell thickness are fabricated with poly(lactic-co-glycolic acid) (PLGA) with varying molecular weights and compositions. The microcapsules show a remarkably high drug encapsulation efficiency of nearly 100% and a high loading capacity (8900 μg siRNA/1 mg polymer). Additionally, microcapsules with an average diameter of 40 μm show superior syringeability when tested with needles ranging from gauge sizes of 27 to 32 G. This makes them suitable for various injection routes. Two sustained-release formulations were selected based on a 3-month in vitro release test. Subsequently, these formulations were injected subcutaneously into mice to verify their in vivo release profiles. The findings demonstrate that the microcapsules effectively shield the siRNAs from being cleared and enable them to be released constantly over 3 months. In contrast, unencapsulated siRNAs are rapidly cleared.
用于持续、局部和可控siRNA递送的可注射微胶囊
临床使用小干扰RNA (siRNA)和反义寡核苷酸通常需要侵入性给药途径,包括鞘内或眼内注射。此外,这些治疗往往需要反复注射。虽然纳米颗粒配方和化学修饰延长了siRNA的治疗持久性,但挑战仍然存在,例如大剂量注射具有高毒性的副作用和急性期最大暴露。我们提出了一种基于微胶囊的方法来局部扩展胆固醇偶联siRNA的活性。利用微流体技术,用不同分子量和组成的聚乳酸-羟基乙酸(PLGA)制备了具有明确尺寸分布和壳厚的微胶囊。该微胶囊具有接近100%的药物包封率和较高的载药量(8900 μg siRNA/ 1mg聚合物)。此外,平均直径为40 μm的微胶囊在27至32 g的针径范围内测试时显示出优越的注射性,这使得它们适用于各种注射途径。通过3个月体外释放试验,选择两种缓释制剂。随后,将这些制剂皮下注射到小鼠体内以验证其体内释放谱。研究结果表明,微胶囊有效地保护sirna不被清除,并使其在3个月内持续释放。相反,未封装的sirna被迅速清除。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


