Rongalite as a Methylene Surrogate: Synthesis of Heterodiarylmethanes via C(sp2)-H Functionalization
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-12-02
DOI:10.1021/acs.joc.4c0214310.1021/acs.joc.4c02143
引用次数: 0
Abstract
An efficient method for the synthesis of heterodiarylmethanes through the coupling of imidazo[1,2-a]pyridines and heteroarenes using indoles employing rongalite as a methylenating reagent has been developed. This regioselective C–H functionalization provides a wide range of heterodiarylmethanes of imidazo[1,2-a]pyridines and imidazo[2,1-b]thiazole. Here, rongalite plays a crucial role in generating a C1 unit in situ, which triggers the heterodiarylmethylation process. The use of inexpensive rongalite (ca. $0.03/1 g), mild reaction conditions, and gram-scale synthesis are some of the key features of this methodology.
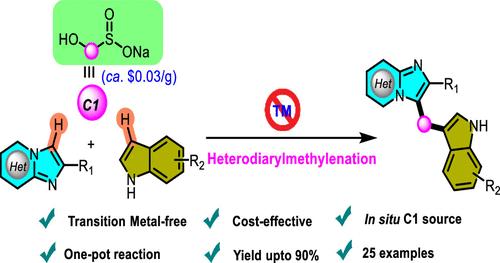
作为亚甲基替代物的熔铝石:C(sp2)-H功能化合成杂二芳基甲烷
本研究开发了一种以吲哚为甲基化试剂,通过咪唑并[1,2-a]吡啶和杂环戊烯的偶联合成杂二芳基甲烷的高效方法。这种区域选择性 C-H 功能化提供了咪唑并[1,2-a]吡啶和咪唑并[2,1-b]噻唑的多种杂二芳基甲烷。在这里,菱镁矿在原位生成 C1 单元,从而引发杂二芳基甲基化过程中发挥了至关重要的作用。使用廉价的菱镁矿(约 0.03 美元/1 克)、温和的反应条件和克级规模的合成是该方法的一些主要特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


