One-Pot Aminomethylation of Heteroarenes by Rongalite as In Situ C1 Source
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-12-04
DOI:10.1021/acs.joc.4c0205010.1021/acs.joc.4c02050
引用次数: 0
Abstract
Rongalite-mediated one-pot aminomethylation of heteroarenes using secondary amines/anilines has been developed. This transition-metal-free and mild reaction offers an efficient way to synthesize aminomethylated heteroaromatic compounds with high yields and broad functional group tolerance. Here, Rongalite plays a key role in generating the C1 unit source in situ, which triggers the aminomethylation process. This approach provides a library of aminomethylated imidazo[1,2-a]pyridines and imidazo[2,1-b]thiazoles.
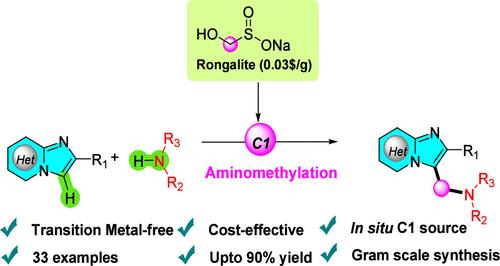
容铝石原位C1源对杂芳烃的一锅氨基甲基化反应
以仲胺/苯胺为原料,荣格酸盐介导杂芳烃的一锅胺甲基化反应。该反应温和、无过渡金属,为合成收率高、官能团耐受性广的氨基甲基化杂芳烃化合物提供了有效途径。在这里,Rongalite在原位生成C1单元源中起着关键作用,这触发了氨基甲基化过程。这种方法提供了一个氨基甲基化咪唑[1,2-a]吡啶和咪唑[2,1-b]噻唑的文库。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


