Asymmetric Synthesis of P-Chirogenic Ferrocenyl BiphePFc* Diphosphine by Ephedrine–Aryne Methods and Application in Rhodium-Catalyzed Hydrogenation
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-12-02
DOI:10.1021/acs.joc.4c0239110.1021/acs.joc.4c02391
引用次数: 0
Abstract
Chiral diphosphines with a biphenyl bridge and the chirality borne by the phosphorus atoms and not due to the atropoisomery of the biaryl backbone have been scarcely studied. Herein, we report the asymmetric synthesis of the (S,S)-2,2′-bis(ferrocenylphenylphosphino)biphenyl (BiphePFc*) ligand and its application in Rh-catalyzed hydrogenation. The synthesis was based on the enantioselective preparation of P-chirogenic ferrocenyl(o-bromophenyl)phenylphosphine by the reaction of sec-phosphine-borane with 1,2-dibromobenzene and its homocoupling into BiphePFc*. Hydrogenations catalyzed by the Rh/BiphePFc* complex led to enantioselectivities of ≤96%.
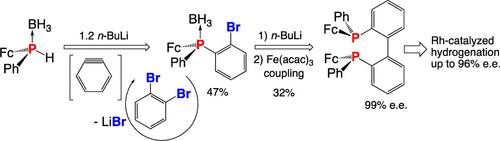
麻黄碱-芳烃法不对称合成对氮源二茂铁基BiphePFc*二膦及其在铑催化加氢中的应用
具有联苯基桥的手性二膦以及由磷原子携带而不是由联芳基主链的反作用力引起的手性的研究很少。本文报道了不对称合成(S,S)-2,2 ' -双(二茂铁苯基膦)联苯(BiphePFc*)配体及其在铑催化加氢中的应用。该合成基于对映选择性制备对氮源二茂铁(邻溴苯基)苯基膦,该二茂铁硼烷与1,2-二溴苯反应并偶联成BiphePFc*。由Rh/BiphePFc*络合物催化的氢化反应导致对映选择性≤96%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


