Photoinduced Aromatization-Driven Deconstructive Fluorosulfonylation of Spiro Dihydroquinazolinones
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-30
DOI:10.1021/acs.joc.4c0230410.1021/acs.joc.4c02304
引用次数: 0
Abstract
A catalyst-free photoinduced deconstructive fluorosulfonylation cascade of spiro dihydroquinazolinones with DABSO and NFSI is reported. This protocol features mild reaction conditions, good yields and excellent functional group tolerance, providing a practical approach to the quinazolin-4(1H)-one-functionalized aliphatic sulfonyl fluorides. In addition, the ease of gram-scale synthesis and the versatility of the SuFEx exchange highlight the application potential of this protocol.
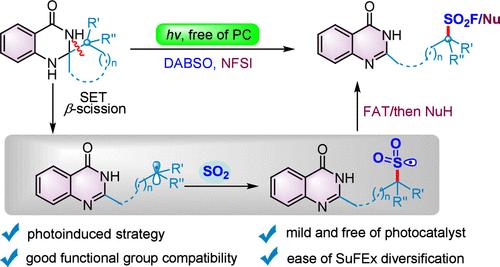
光诱导芳构化驱动的螺旋二氢喹唑啉酮的解构氟磺化
报告了一种无催化剂光诱导的螺二氢喹唑啉酮与 DABSO 和 NFSI 的解构性氟磺酰化级联反应。该方案反应条件温和、产率高、官能团耐受性好,为喹唑啉-4(1H)-酮官能化脂肪族磺酰氟提供了一种实用的方法。此外,克级合成的简易性和 SuFEx 交换的多功能性也凸显了该方案的应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


