Amphiphilic Photoluminescent Porous Silicon Nanoparticles as Effective Agents for Ultrasound-Amplified Cancer Therapy
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
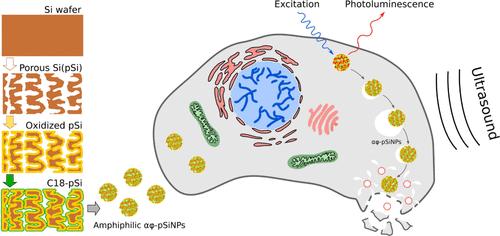
This study investigates the use of photoluminescent amphiphilic porous silicon nanoparticles (αϕ-pSiNPs) as effective ultrasound (US) amplifiers for cancer sonodynamic theranostics. αϕ-pSiNPs were synthesized via a novel top-down approach involving porous silicon (pSi) films electrochemical etching, borate oxidation, and hydrophobic coating with octadecylsilane (C18), resulting in milling into nanoparticles with hydrophilic exteriors and hydrophobic interiors. These properties promote gas trapping and cavitation nucleation, significantly lowering the US cavitation threshold and resulting in selective destruction of cancer cells in the presence of nanoparticles. Efficient internalization of αϕ-pSiNPs in cell cytoplasm was demonstrated by their intrinsic photoluminescence, activated by partial oxidation of mesoporous silicon films in borate solutions, which resulted in quantum confinement of excitons in 2–5 nm Si quantum dots/wires. Combined with US exposure above the cavitation threshold, αϕ-pSiNPs caused a significant decrease in cell viability through mechanical stretching and microflows generated by oscillating microbubbles. Meanwhile, αϕ-pSiNPs exhibit high biocompatibility up to concentrations of 1 mg/mL without US activation. Their photoluminescent properties facilitate bioimaging, while their US contrast capabilities may enhance both imaging and therapy. The dual functionality of αϕ-pSiNPs supports a theranostic approach, enabling simultaneous diagnostics and treatment with a single agent. This study underscores the potential of αϕ-pSiNPs in sonodynamic therapy and bioimaging, offering a promising strategy for effective and safe anticancer therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


