Seeding Control in Chirality Triggering of Red-Emitting Organic Charge-Transfer Cocrystal Helixes from Achiral Molecules
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
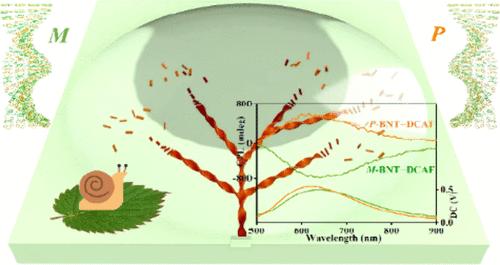
Supramolecular chirality has gained immense attention for great potential, in which the rational engineering strategy facilitates unique helical stacking/assembly, high chiroptical behavior, and prime biomedical activity. In this study, we reported a novel chiral organic donor–acceptor cocrystal based on asymmetrical components of benzo(b)naphtho(1,2-d)thiophene (BNT) and 9-oxo-9H-indeno(1,2-b)pyrazine-2,3-dicarbonitrile (DCAF) that exhibited red emission using a simple solution approach. During the self-assembly, a kinetically controlled growth of polar solvent or substrate induction led to the chiral packing and helical morphology twisted by the cooperation of electrostatic potential energy and chirality. Intriguingly, a “seeding-control” mechanism was newly developed for the production of c-BNT–DCAF helical crystals with a defined uniform chiral form, which enables chirality transfer and amplification from the microscopic to macroscopic level via supramolecular stacking. By introducing chiral additives or even a small break at the edge, the first nucleus acted as a chiral seeding to guide the donor/acceptor molecule alignment into the same handedness. A remarkably high dissymmetry factor (glum) value of 0.1 was demonstrated on the chiral manipulated ribbons, which is the highest among the reported charge-transfer complexes. This work offers us more paths for the design of chiral supramolecular systems for vital applications in organic optoelectronics, micro/nanomechanics, and biomimetics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


