Capture and Diffusion of Hydrogen in Tantalum and Copper with Vacancy Defects: A First-Principles Study
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
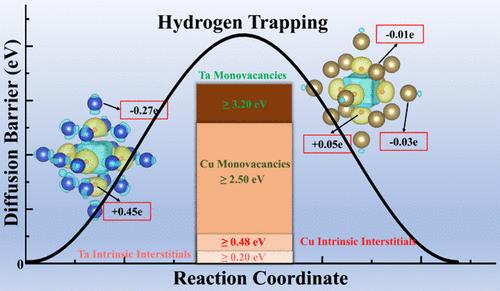
Oxygen-free copper is utilized in nuclear processing heaters; however, it exhibits poor resistance to hydrogen radiation corrosion. A tantalum–copper diffusion layer with high vacancy concentration was prepared on the copper surface. This layer demonstrates superior hydrogen trapping and diffusion resistance compared to pure tantalum, though the underlying mechanism remains unclear. First-principles DFT methods were employed to investigate the absorption of hydrogen atoms by tantalum and copper vacancies, forming vacancy-hydrogen complexes, and their diffusion characteristics. These were compared with interstitial configurations. The ground state formation energy is lowest when a tantalum vacancy captures six hydrogen atoms. It can accommodate up to 12 hydrogen atoms while maintaining a higher energy than the interstitial configuration, forming a spherical structure with special symmetry. For copper vacancies, the formation energy remains higher than the interstitial configuration when capturing up to six hydrogen atoms. The high-vacancy diffusion layer exhibits a strong hydrogen trapping capacity. Posthydrogen capture, the overall migration energy for both tantalum and copper vacancies exceeds 2.5 eV. The energy barrier for individual hydrogen atom diffusion outward is higher than in interstitial cases when capturing up to six hydrogen atoms. Vacancies capturing hydrogen atoms play a role in maintaining the stability of hydrogen in its ground state.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


