Merging photoredox with metalloenzymatic catalysis for enantioselective decarboxylative C(sp3)‒N3 and C(sp3)‒SCN bond formation
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The scope of nature’s catalytic abilities has been expanded by recent advancements in biocatalysis to include synthetic transformations with no biological equivalent. However, these newly introduced catalytic functions represent only a small fraction of reactions utilized in synthetic catalysis. Here we present a biocatalytic platform that combines photoredox and metalloenzymatic catalysis for enantioselective radical transformations. Under green light irradiation, the eosin Y photocatalyst enables 4-hydroxyphenylpyruvate dioxygenases to catalyse enantioselective decarboxylative azidation and thiocyanation of N-hydroxyphthalimide esters. The final optimized variant obtained through directed evolution can afford diverse chiral organic azide and thiocyanate compounds with up to 77% yield, 385 total turnovers and 94% enantiomeric excess. Mechanistic studies show that the eosin Y catalyst mediates the generation of both C(sp3) radical and Fe(III)‒N3/Fe(III)‒NCS intermediate, leading to efficient enantioselective C‒N3 and C‒SCN bond formation in the enzyme active site. These findings establish an adaptable biocatalytic platform for introducing abiological metallophotoredox catalysis into biology. Decarboxylative azidation is a valuable transformation in organic chemistry, but a biocatalytic equivalent remained elusive. Now merging photoredox with metalloenzymatic catalysis enables the enantioselective decarboxylative radical azidation and thiocyanation of N-hydroxyphthalimide esters.
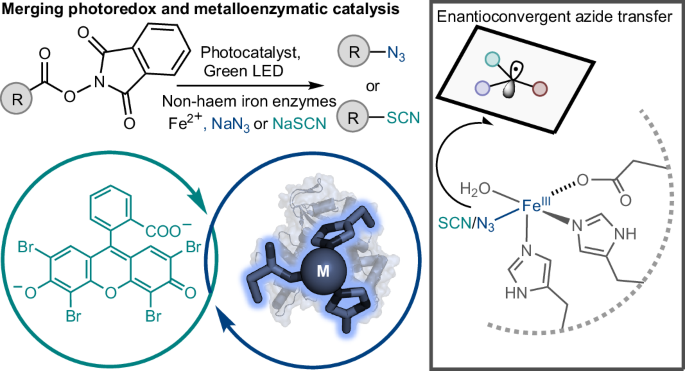

合并光氧化还原与金属酶催化对映选择性脱羧C(sp3) -N3和C(sp3) -SCN键形成
自然的催化能力的范围已经扩大了生物催化的最新进展,包括合成转化没有生物等价物。然而,这些新引入的催化功能只代表了合成催化中使用的反应的一小部分。在这里,我们提出了一个结合光氧化还原和金属酶催化对映选择性自由基转化的生物催化平台。在绿光照射下,伊红Y光催化剂使4-羟基苯基丙酮酸双加氧酶催化n -羟基邻苯亚胺酯的对映选择性脱羧叠氮化和硫氰化。通过定向进化获得的最终优化变体可以产生多种手性有机叠氮化物和硫氰酸酯化合物,产率高达77%,总周转率为385,对映体过量为94%。机制研究表明,伊红Y催化剂同时介导C(sp3)自由基和Fe(III) -N3 /Fe(III) -NCS中间体的生成,从而在酶活性位点形成高效的对映选择性C -N3和C - scn键。这些发现为将生物金属光氧化还原催化引入生物学领域建立了一个适应性强的生物催化平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


