Promoted *OH Adsorption Facilitates C–C Bond Cleavage for Efficient Electrochemical Upcycling of Polyethylene Terephthalate
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
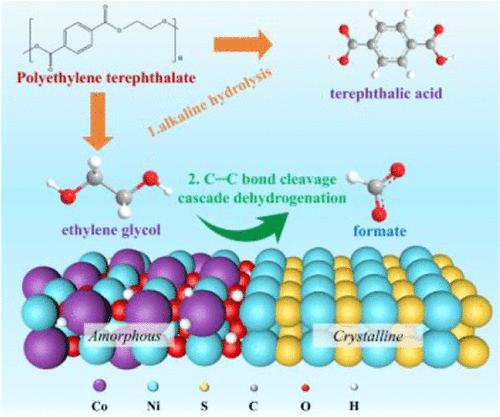
The electrochemical oxidation of ethylene glycol (EG) derived from polyethylene terephthalate (PET) plastic into value-added chemicals, coupled with hydrogen evolution, offers a promising approach to addressing plastic pollution. However, the mechanisms by which the adsorption of key reaction intermediates affects the EG oxidation reaction (EGOR) are not well understood. To investigate this, we synthesized two model catalysts: amorphous-phase CoNiOOH/NF and CoNiOOH–Ni3S2/NF with an amorphous/crystalline interface. Detailed characterizations and theoretical calculations demonstrate that the amorphous/crystalline interface in CoNiOOH–Ni3S2/NF shifts the d-band center upward, enhancing the adsorption of EG and *OH compared to amorphous CoNiOOH/NF. Enhanced *OH adsorption is crucial for promoting C–C bond cleavage and subsequent dehydrogenation. In situ electrochemical infrared absorption spectroscopy (IRAS) and theoretical calculations reveal that formate (FA) is primarily formed through cleavage of the C–C bond in glycolic acid, followed by oxidation. Notably, CoNiOOH–Ni3S2/NF achieves industrial-level current densities of 500 mA cm–2 at an ultralow potential of 1.45 V vs RHE, with a Faradaic efficiency (FE) of 96.6% and FA productivity of 3.14 mmol cm–2 h–1 at 1.70 V vs RHE. This study offers valuable insights for designing efficient heterojunction catalysts for the electrochemical upcycling of PET plastics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


