Advances in the selective c-MET kinase inhibitors: Application of fused [5,6]-Bicyclic nitrogen-containing cores for anticancer drug design
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Over the past two decades, small molecules bearing [5,6]-bicyclic nitrogen-containing cores have emerged as one of the most extensively studied structures for the development of selective c-MET kinase inhibitors. Structure-activity relationship (SAR) studies have demonstrated that modifying these cores can significantly impact the biological properties of c-MET inhibitors, including safety/toxicity, potency, and metabolic stability. For example, although c-MET kinase inhibitors containing the [1,2,4]triazolo[4,3-b][1,2,4]triazine scaffold (core P) exhibit high inhibitory potency, they often face challenges due to metabolic stability defects. Alternatively, compounds containing [1,2,3]triazolo[4,5-b]pyrazine (core K) and [1,2,4]triazolo[4,3-b]pyridazine (core I) scaffolds demonstrate lower potency but improved metabolic stability, allowing some of them to progress into clinical trials and even be approved as novel anticancer drugs. Fortunately, X-ray crystallography studies have well elucidated key interactions between [5,6]-bicyclic nitrogen-containing cores and crucial amino acid residues within the c-MET active site. These insights emphasize the significance of π-π stacking interactions with Tyr1230 and hydrogen bonding with Asp1222, providing valuable guidance for the targeted design and optimization of selective c-MET kinase inhibitors. Following the identification/introduction of sixteen distinct [5,6]-bicyclic nitrogen-containing cores (cores A-P) utilized in the design of selective c-MET kinase inhibitors over the past two decades, this manuscript offers a comprehensive review of their roles in drug development of anticancer agents, and describes the various synthesis methods employed. The insights presented herein can serve as a resource for better structural optimization of c-MET kinase inhibitors in the future research.
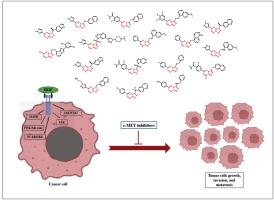

选择性c-MET激酶抑制剂的研究进展:融合[5,6]-双环含氮核心在抗癌药物设计中的应用
在过去的二十年里,承载[5,6]-双环含氮核心的小分子已经成为开发选择性c-MET激酶抑制剂的最广泛研究的结构之一。构效关系(SAR)研究表明,修改这些核心可以显著影响c-MET抑制剂的生物学特性,包括安全性/毒性、效力和代谢稳定性。例如,虽然含有[1,2,4]三唑[4,3-b][1,2,4]三嗪支架(核心P)的c-MET激酶抑制剂表现出很高的抑制效力,但由于代谢稳定性缺陷,它们经常面临挑战。另外,含有[1,2,3]三唑[4,5-b]吡嗪(核心K)和[1,2,4]三唑[4,3-b]吡嗪(核心I)支架的化合物药效较低,但代谢稳定性较好,使其中一些化合物进入临床试验,甚至被批准为新型抗癌药物。幸运的是,x射线晶体学研究已经很好地阐明了[5,6]-双环含氮核与c-MET活性位点内关键氨基酸残基之间的关键相互作用。这些发现强调了π-π堆叠相互作用与Tyr1230和氢键与Asp1222的重要性,为选择性c-MET激酶抑制剂的靶向设计和优化提供了有价值的指导。在过去二十年中,在选择性c-MET激酶抑制剂的设计中使用了16种不同的[5,6]-双环含氮核心(核心a - p),本文对它们在抗癌药物开发中的作用进行了全面回顾,并描述了所采用的各种合成方法。本文提出的见解可以作为未来研究中更好地优化c-MET激酶抑制剂结构的资源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


