Multiple Binding Modes of Inhibitors to Human Carbonic Anhydrases: An Update on the Design of Isoform-Specific Modulators of Activity
IF 55.8
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
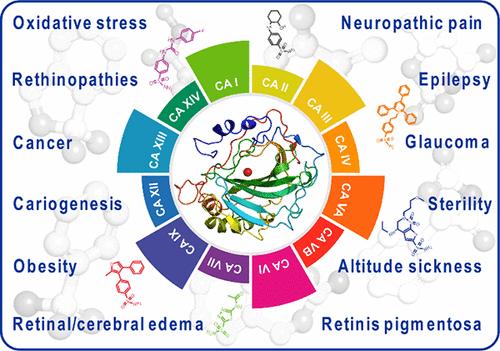
Human carbonic anhydrases (hCAs) are widespread zinc enzymes that catalyze the hydration of CO2 to bicarbonate and a proton. Currently, 15 isoforms have been identified, of which only 12 are catalytically active. Given their involvement in numerous physiological and pathological processes, hCAs are recognized therapeutic targets for the development of inhibitors with biomedical applications. However, despite massive development efforts, very few of the presently available hCA inhibitors show selectivity for a specific isoform. X-ray crystallography is a very useful tool for the rational drug design of enzyme inhibitors. In 2012 we published in Chemical Reviews a highly cited review on hCA family (
人碳酸酐酶抑制剂的多种结合模式:同型特异性活性调节剂设计的最新进展
人体碳酸酐酶(hCAs)是一种广泛存在的锌酶,可催化二氧化碳水合转化为碳酸氢盐和质子。目前已发现 15 种同工酶,其中只有 12 种具有催化活性。由于 hCAs 参与了许多生理和病理过程,因此是开发生物医学应用抑制剂的公认治疗目标。然而,尽管进行了大量的开发工作,目前可用的 hCA 抑制剂中只有极少数对特定的同工酶具有选择性。X 射线晶体学是合理设计酶抑制剂的有用工具。2012 年,我们在《化学评论》(Chemical Reviews)上发表了一篇关于 hCA 家族的综述(Alterio, V. et al. Chem Rev. 2012, 112, 4421-4468),分析了约 300 种 hCA/抑制剂复合物的晶体学结构,并描述了迄今为止存在的不同 CA 抑制机制,文章的引用率很高。然而,在 2012-2023 年期间,已有近 700 个新的 hCA/抑制剂复合物结构存入 PDB,并发现了大量新的抑制剂类别。基于这些考虑,本综述旨在全面介绍 2012-2023 年间 hCA/抑制剂相互作用结构方面的最新进展,并概述如何利用这些信息合理设计更具选择性的此类抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


