Electrochemical Chlorozincate Anion Intercalation into Layered Carbon Materials for the Cathodes of Aqueous Zinc Metal Secondary Batteries
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Aqueous zinc metal secondary batteries (ZSBs) are expected to be next-generation secondary batteries, and it is important to explore cathode materials and electrolyte solutions that exhibit excellent electrochemical properties for their practical use. In this study, we employed a layered carbon material named graphene-like graphite (GLG) as a cathode active material and a concentrated aqueous zinc chloride solution as an electrolyte solution, and its electrochemical anion intercalation reaction was investigated. As a result, GLG obtained at 300 °C of thermal treatment (GLG300) exhibited lower anion intercalation potential and better Coulombic efficiency in ZnCl2·2.33H2O compared to graphite and GLG obtained at 700 °C. X-ray diffraction measurement suggested that GLG300 formed a stage-1 intercalation compound at 1.8 V vs Zn2+/Zn, and extended X-ray absorption fine structure analysis revealed that the intercalated anion was hydrated [ZnCl4]2–. The initial discharge capacity of GLG300 was approximately 170 mAh g–1 in the potential range of 0.5–2.2 V with a current density of 20 mA g–1. The charge–discharge cycling test showed that GLG300 had good reversibility, the discharge capacity remained above 110 mAh g–1, and the Coulombic efficiency approached nearly 100% at the 50th cycle. These results demonstrated that the system using GLG300 and concentrated aqueous zinc chloride solution exhibits excellent cathode properties as aqueous ZSBs and showed great promise for their future practical use.
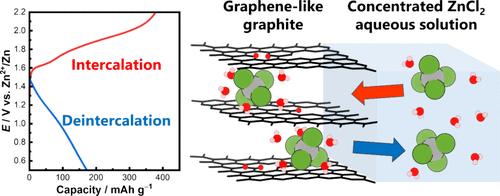
氯锌酸盐负离子在层状碳材料中的电化学嵌入
水锌金属二次电池有望成为下一代二次电池,探索具有优异电化学性能的正极材料和电解质溶液对其实际应用具有重要意义。本研究以层状碳材料石墨烯样石墨(GLG)为正极活性材料,以浓氯化锌水溶液为电解质溶液,研究其电化学阴离子插层反应。结果表明,300℃热处理得到的GLG (GLG300)在ZnCl2·2.33H2O中表现出较低的阴离子嵌入电位和较好的库仑效率。x射线衍射测试表明,在1.8 V vs Zn2+/Zn下,GLG300形成了1级插层化合物,扩展x射线吸收精细结构分析表明,插层阴离子为水合[ZnCl4]2 -。在0.5-2.2 V电压范围内,电流密度为20 mA g-1, GLG300的初始放电容量约为170 mAh g-1。充放电循环试验表明,GLG300具有良好的可逆性,放电容量保持在110 mAh g-1以上,第50次循环时库仑效率接近100%。这些结果表明,GLG300与浓氯化锌水溶液组成的体系具有良好的正极性能,具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


