Factors affecting capacity and voltage fading in disordered rocksalt cathodes for lithium-ion batteries
IF 17.5
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Disordered rocksalt cathodes deliver high energy densities, but they suffer from pronounced capacity and voltage fade on cycling. Here, we investigate fade using two disordered rocksalt lithium manganese oxyfluorides: Li3Mn2O3F2 (Li1.2Mn0.8O1.2F0.8), which stores charge by Mn2+/Mn4+ redox, and Li2MnO2F, where charge storage involves both Mn3+/Mn4+ and oxygen redox (O-redox). Li3Mn2O3F2 is reported for the first time. We identify the growth of an electronically resistive surface layer with cycling that is present in both Li2MnO2F and Li3Mn2O3F2 but more pronounced in the presence of O-redox. This resistive surface inhibits electronic contact between particles, leading to the observed voltage polarization and capacity loss. By increasing carbon loading in the composite cathode, it is possible to substantially improve the cycling performance. These results help to disentangle O-redox from other leading causes of capacity fading in Mn oxyfluorides and highlight the importance of maintaining electronic conductivity in improving capacity and voltage retention.
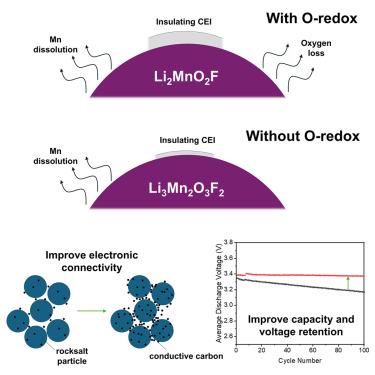

影响锂离子电池无序岩盐阴极容量和电压衰减的因素
无序岩盐阴极提供高能量密度,但它们在循环时受到明显的容量和电压衰减的影响。在这里,我们使用两种无序的岩盐氧化锰锂氟化物:Li3Mn2O3F2 (Li1.2Mn0.8O1.2F0.8),它通过Mn2+/Mn4+氧化还原储存电荷,Li2MnO2F,其中电荷存储涉及Mn3+/Mn4+和氧氧化还原(o -氧化还原)。Li3Mn2O3F2为首次报道。我们发现在Li2MnO2F和Li3Mn2O3F2中都存在一个电子电阻表面层的循环生长,但在o -氧化还原的存在下更为明显。这种电阻表面抑制粒子之间的电子接触,导致观察到的电压极化和容量损失。通过增加复合阴极的碳负荷,有可能大幅度提高循环性能。这些结果有助于将o -氧化还原从氧氟化锰中容量衰退的其他主要原因中分离出来,并强调了保持电子导电性在提高容量和电压保持方面的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Matter
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
26.30
自引率
2.60%
发文量
367
期刊介绍:
Matter, a monthly journal affiliated with Cell, spans the broad field of materials science from nano to macro levels,covering fundamentals to applications. Embracing groundbreaking technologies,it includes full-length research articles,reviews, perspectives,previews, opinions, personnel stories, and general editorial content.
Matter aims to be the primary resource for researchers in academia and industry, inspiring the next generation of materials scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


