Overcoming Gas Mass Transfer Limitations Using Gas-Conducting Electrodes for Efficient Nitrogen Reduction
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
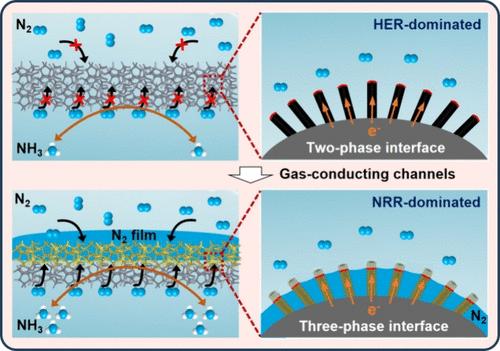
Electrocatalytic nitrogen reduction reaction (NRR) is a very attractive strategy for ammonia synthesis due to its energy savings and sustainability. However, the ammonia yield and Faraday efficiency of electrocatalytic nitrogen reduction have been challenges due to low nitrogen solubility and competitive hydrogen evolution reaction (HER) in electrolyte solution. Herein, inspired by the asymmetric wetting behavior, i.e., superhydrophobicity/hydrophilicity, of floating lotus leaves, we demonstrated a gas-conduction electrode with asymmetric gas wetting behavior on the opposite surface, i.e., Janus-Ni/MoO2@NF, for efficient nitrogen reduction. It can provide an abundant three-phase interface (TPI) at interfaces of Janus-Ni/MoO2@NF in electrolyte solution to enhance the contact among N2, electrolyte, and electrode. Ascribed to this advantage, the hydrophobic side of the Janus electrode not only can repel water molecules to suppress the HER process but also can increase the concentration of N2 on the interface microenvironment. Consequently, the well-designed gas-conducting electrode breaks gas mass transfer limitation. Furthermore, Janus-Ni/MoO2@NF delivers a record-high NH3 yield rate of 5.865 μg·h–1·cm–2 and a Faradaic efficiency of 36.14% at an extremely low potential of 0 V vs RHE in 0.1 M Na2SO4 under ambient conditions, which are 22 and 18 times higher than those of the conventional electrode, respectively. Therefore, the gas-conducting electrodes can dramatically improve the activity and selectivity in electrocatalytic NRR. Additionally, the unique interface design provides inspiration for other sustainable electrochemical reactions involving gas electrocatalytic correlation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


