Alkali metal cations act as homogeneous cocatalysts for the oxygen reduction reaction in aqueous electrolytes
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Alkali metal cations (AM+) exhibit high solubility and ionic conductivity, making them optimal components in aqueous electrolytes. Despite the conventional belief that AM+ are chemically inert spectators, the strong dependence of electrocatalysis on AM+ has recently provoked debates about their unforeseen catalytic role. However, conclusive evidence is still lacking. Here we demonstrate that AM+ can couple with reaction intermediates and determine kinetics as homogeneous cocatalysts in aqueous conditions, for the alkaline oxygen reduction reaction on a carbon catalyst. In situ X-ray absorption spectroscopy reveals a change in the electronic structure of Na+ from its hydrated state on a charged electrode. In situ Raman spectroscopy further identifies that this change is due to the formation of water-unstable NaO2 as a key intermediate in OOH− production. Together with theoretical calculations, this finding enunciates the counterintuitive cocatalytic role of AM+ in aqueous environments, highlighting the exigency of refined interface design principles for better electrocatalysis. Alkali cations in electrolytes are commonly considered chemically inert species, but their role has recently been called into question. Now, using in situ spectroscopy and molecular dynamics simulations, it is shown that alkali cations couple with intermediates in the oxygen reduction reaction, acting as cocatalysts.
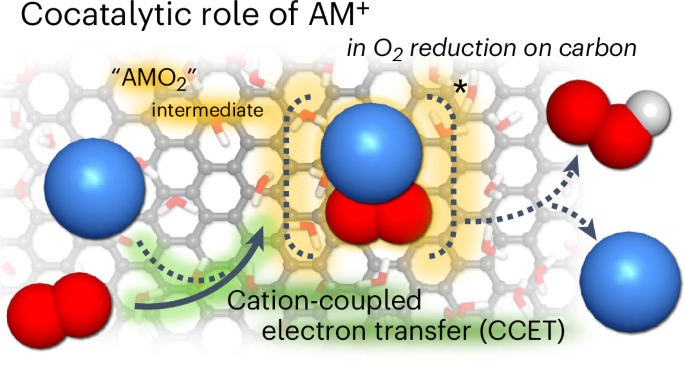

碱金属阳离子是水溶液中氧还原反应的均相助催化剂
碱金属阳离子(AM+)具有较高的溶解度和离子导电性,使其成为水性电解质的最佳成分。尽管传统上认为AM+是化学惰性的旁观者,但电催化对AM+的强烈依赖最近引发了关于其不可预见的催化作用的争论。然而,目前仍缺乏确凿的证据。在这里,我们证明了AM+可以与反应中间体偶联,并确定了在水条件下作为均相助催化剂在碳催化剂上进行碱性氧还原反应的动力学。原位x射线吸收光谱揭示了Na+在带电电极上水合状态下电子结构的变化。原位拉曼光谱进一步确定了这种变化是由于水不稳定的NaO2的形成,NaO2是OOH -生产的关键中间体。结合理论计算,这一发现阐明了AM+在水环境中的反直觉共催化作用,强调了改进界面设计原则以获得更好的电催化的紧迫性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


