Kinetics for Co catalyzed oxidative cyanation of biomass-based furfural
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
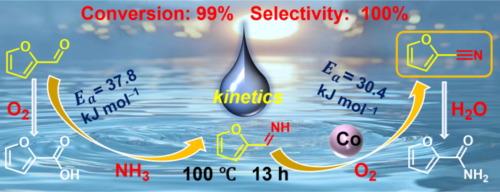
Oxidative cyanation of furfural (1a) to 2-furancarbonitrile (3a) by using NH3 as a nitrogen source and O2 as an oxidant is an effective strategy for biomass-based nitrile synthesis. Herein, Co catalyst supported on a nanocomposite of nitrogen-doped carbon and TiO2 (Co/NC-TiO2) was developed for the oxidative cyanation. The multiphase interface architecture of the catalyst enriched oxygen vacancies; moreover, the electron-rich NC nanocomponent facilitated Co2+/Co3+ valence transformation, thus promoting O2 activation. The kinetic analysis demonstrated the condensation of 1a/NH3-to- (2-furanyl)methanimine (2a) as the rate-determining step, which was consecutively promoted by 2a/O2-to-3a dehydrogenation over the catalyst surface. A Langmuir-Hinshelwood mechanism was suggested for the oxidative dehydrogenation of the 2a/O2-to-3a step, in which O2 is activated by an associative adsorption on the Co surface yielding a superoxide radical (O2•–) species for 2a dehydrogenation with the release of H2O2. This research highlights a kinetic and mechanic understanding of the catalytic oxidative cyanation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


