One-Pot Chemoenzymatic Synthesis of Arsinothricin and the Mechanistic Insights into the Noncanonical Radical SAM Enzyme ArsL
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Arsinothricin (AST) is a broad-spectrum arsenic-containing antibiotic with promising pharmaceutical properties. In this study, we report the one-pot chemoenzymatic synthesis of AST starting from methylarsenate, a commonly used agricultural herbicide. Although a single point mutation in the C-terminal region of ArsL completely abolished its activity toward the natural substrate inorganic arsenite, this mutation unexpectedly enhanced its activity toward methylarsenate by over 50-fold, enabling subgram scale production of AST in a cell-free system. These findings offer valuable mechanistic insights into ArsL and highlight the significant potential of manipulating the radical SAM superfamily enzymes in synthetic applications.
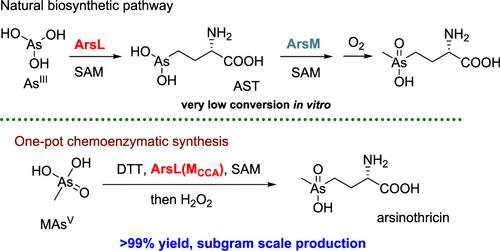
Arsinothricin (AST) 是一种广谱含砷抗生素,具有广阔的药用前景。在本研究中,我们报告了以常用的农用除草剂甲胂酸为原料,一步化学合成 AST 的过程。虽然 ArsL C 端区域的单点突变完全取消了其对天然底物无机亚砷酸盐的活性,但这一突变却意外地将其对甲砷酸盐的活性提高了 50 倍以上,从而实现了在无细胞系统中亚克规模生产 AST。这些发现为 ArsL 提供了宝贵的机理启示,并凸显了在合成应用中操纵自由基 SAM 超家族酶的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


