Enhancement of Methylene Blue Adsorption by Acid–Base Neutralization-Induced Bulging MXene/RGO Composite Foams
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nanocomposite films made from graphene oxide (GO) and MXene have a dense layered structure due to nanosheet self-stacking, limiting their dye adsorption performance. In this study, acid–base neutralization reactions are used to induce MXene/reduced graphene oxide (RGO) films bulging, which opens the stacked layer structure within the membrane and enhances MB adsorption performance. The effects of the pH, temperature, contact time, and initial concentration of MB on the adsorption performance are further investigated. The results indicate that the adsorption process conforms to the pseudo-second-order kinetic and Freundlich isotherm models and is heat-absorbing and spontaneous, and the MXene/RGO foams have an adsorption capacity of up to 1099.5 mg g–1 for MB. In addition, our study show that the MXene/RGO foams not only have better reusability, but also exhibit better adsorption for other dyes. The efficient MB removal is attributed to the increased specific surface area of the composite foams, increased active sites, strong electrostatic interactions between MB and the composite foams, as well as intercalation adsorption. These findings offer new options for solving dye effluent problems.
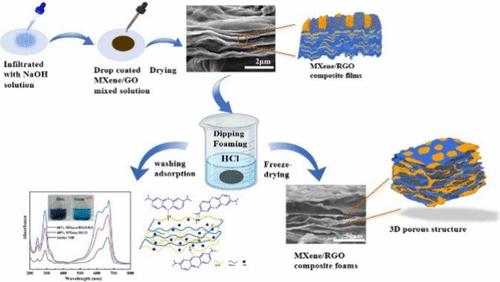
酸碱中和诱导膨胀MXene/RGO复合泡沫增强对亚甲基蓝的吸附
由氧化石墨烯(GO)和MXene制成的纳米复合膜由于纳米片的自堆叠而具有致密的层状结构,限制了其染料吸附性能。本研究利用酸碱中和反应诱导MXene/还原氧化石墨烯(RGO)薄膜膨胀,打开膜内的堆叠层结构,提高MB吸附性能。进一步考察了pH、温度、接触时间和MB初始浓度对吸附性能的影响。结果表明,MXene/RGO泡沫对MB的吸附量可达1099.5 mg g-1。MXene/RGO泡沫不仅具有较好的可重复使用性,而且对其他染料也有较好的吸附能力。复合泡沫的比表面积增加,活性位点增加,MB与复合泡沫之间的强静电相互作用以及插层吸附是有效去除MB的原因。这些发现为解决染料废水问题提供了新的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
文献相关原料
公司名称
产品信息
阿拉丁
Methyl Orange (MO, C14H14N3NaO3S)
阿拉丁
Methylene Blue (MB, C16H18ClN3S·3H2O)
阿拉丁
NaOH
阿拉丁
Methyl Orange (MO, C14H14N3NaO3S)
阿拉丁
Methylene Blue (MB, C16H18ClN3S·3H2O)
阿拉丁
NaOH
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


