Light-Directed Microalgae Micromotor with Supramolecular Backpacks for Photodynamic Therapy
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
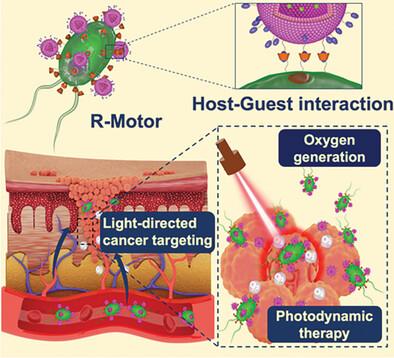
In recent years, the development of micromotors for biomedical applications has surged. However, challenges such as immunogenicity and the difficulty in controlling motion direction have hindered their clinical translation. In this study, Chlamydomonas reinhardtii, a natural unicellular green microalgae known for its biocompatibility and phototaxis properties in hydrogen peroxide environments is applied, as a micromotor for efficient drug delivery and photodynamic therapy. 5-Aminolevulinic acid-loaded liposomes are anchored onto the surface of algae through host–guest complexation between β-cyclodextrin-modified algae and adamantane-modified liposomes. This created a micromotor capable of carrying a drug-loaded backpack for light-driven tumor targeting and drug delivery. Additionally, light irradiation activates the photosynthesis of chloroplasts in microalgae leading to oxygen production and alleviation of the tumor's hypoxic microenvironment. In tumor-bearing mice, light irradiation on tumor tissue directs the micromotor to accumulate in the tumor region, significantly increasing the local drug concentration from 0.007 to 0.069 mg mL−1 compared to free liposomes. Furthermore, oxygen generated from photosynthesis enhances the efficacy of photodynamic therapy, resulting in complete tumor regression in Balb/c mice after 14 days. This system achieves a three-in-one effect, combining targeted drug delivery, oxygen generation, and photodynamic treatment. These findings hold promise for the advancement of micromotor-based biomedical applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


