Stabilizing Diketopyrrolopyrrole Radical Cations Through Carbazoles: Substitution Pattern vs Spin Delocalization
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The synthesis of organic radicals continues to garner significant interest due to their fascinating optical, electronic, and magnetic properties. Moreover, the growing demand for chemically stable organic radicals is driven by the rapid expansion of the market for electronic devices utilizing organic semiconductors. In this context, the development of multifaceted approaches for the design of stable organic radicals is of great importance. In this work, we introduce a strategy for generating stable radical cations of diketopyrrolopyrroles (DPP) by modulating the substitution pattern of the electron-donating carbazole substituent. Using electronic, spin resonance, and vibrational spectroscopies, supported by density functional theory, we carefully investigated the electronic structures and chemical stability of the DPP radical cations. Our findings demonstrate that the position of electron-rich heteroatoms and the presence of Clar’s aromatic sextets in donor moieties play a pivotal role in enhancing the chemical stability of DPP radical cations.
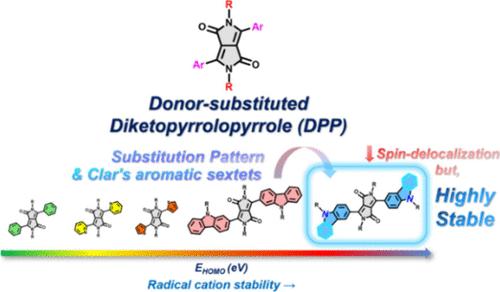
通过咔唑稳定双酮吡咯基阳离子:取代模式与自旋离域
有机自由基的合成由于其迷人的光学、电子和磁性而继续引起人们的极大兴趣。此外,利用有机半导体的电子设备市场的迅速扩大推动了对化学稳定的有机自由基的需求不断增长。在这样的背景下,发展多方位的方法来设计稳定的有机自由基是非常重要的。在这项工作中,我们介绍了一种通过调节给电子咔唑取代基的取代模式来产生稳定的二酮吡咯(DPP)自由基阳离子的策略。利用电子、自旋共振和振动光谱,在密度泛函理论的支持下,我们仔细研究了DPP自由基阳离子的电子结构和化学稳定性。我们的研究结果表明,富电子杂原子的位置和Clar 's芳六元在给体部分的存在对提高DPP自由基阳离子的化学稳定性起关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


