Solvents and their hydrogen bonding properties as general considerations in carbon dioxide reduction by molecular catalysts
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
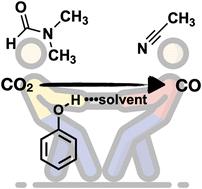
Improvements to the understanding of how reaction conditions influence the performance of molecular electrocatalysts are important. There exists a wide range of solution conditions that are used in the investigation of the properties and performance of electrocatalysts, from the choice of solvent or electrolyte to the identity and nature of other additives, like Brønsted acids. Herein, we demonstrate how the choice of solvent can have a significant impact on the observed rate constants for CO2-to-CO conversion by a series of rhenium(I) diimine complexes. In comparison with the observed rate constants in acetonitrile solvent, the use of a strong hydrogen bond-accepting solvent (N,N-dimethylformamide, DMFf) dramatically decreases the observed rate constants in the presence of added phenol (as a proton donor). Based on previous work from our lab and from others, we conclude that such solvent effects are a general phenomenon and are a crucial consideration for investigation of molecular catalysts. Finally, a simple H-bonding model is presented to account for solvent effects in these rhenium(I) CO2 reduction systems. The model is general for H-bonding solvents and Brønsted acids and provides a first principles means to estimate the magnitude of solvent effects on CO2 reduction kinetics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


