Silver 4,4′-Vinylenedipyridine Coordination Polymers: Linker Effects on Formation Thermodynamics and Anion Exchange
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Four new and one previously reported silver 4,4′-vinylenedipyridine (Vpe) coordination polymers were tested as anion exchange materials to assess their potential for pollutant sequestration and compared to analogous silver 4,4′-bipyridine (bipy) coordination polymers. The materials were synthesized using nitrate, tetrafluoroborate, perchlorate, perrhenate, or chromate as the anion to produce cationic coordination polymers with solubilities ranging from 0.0137(7) to 0.21(5) mM. These values are much lower than silver bipy coordination polymers [0.045(3) to 5.5(5) mM] and agree with thermochemical calculations. [Ag(Vpe)+][BF4–], [Ag2(Vpe)2.52+][CrO42–]·5H2O, and [Ag(Vpe)+][ReO4–]·2H2O structures are reported. Perrhenate and chromate ions in an equimolar solution were fully adsorbed by [Ag(Vpe)+][NO3–]·3H2O [620(2) and 137.1(6) mg/g, respectively] as well as by [Ag(Vpe)+][BF4–] [661.8(3) and 190(3) mg/g, respectively] via anion exchange. DFT calculations show that torsional energetics play a significant role in the formation thermodynamics by reducing the energy cost by as much as 4.8 kJ/mol when bipy is replaced with Vpe in silver-based coordination polymers. The results obtained with the flat Vpe ligand highlight the potential role of coordination polymers in practical anion exchange.
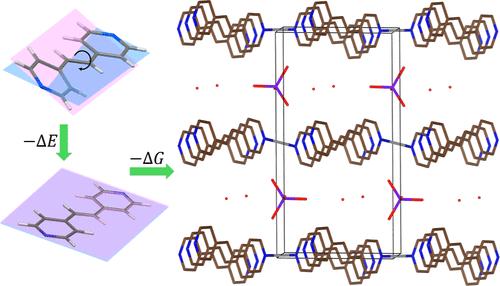
研究人员测试了四种新的和一种以前报道过的 4,4′-乙烯基二吡啶(Vpe)银配位聚合物作为阴离子交换材料,以评估它们螯合污染物的潜力,并与类似的 4,4′-联吡啶(bipy)银配位聚合物进行了比较。这些材料是以硝酸盐、四氟硼酸盐、高氯酸盐、高铼酸盐或铬酸盐作为阴离子合成的阳离子配位聚合物,溶解度在 0.0137(7) 至 0.21(5) mM 之间。这些数值远低于双银配位聚合物[0.045(3) 至 5.5(5) mM],并且与热化学计算结果一致。报告了 [Ag(Vpe)+][BF4-]、[Ag2(Vpe)2.52+][CrO42-]-5H2O 和 [Ag(Vpe)+][ReO4-]-2H2O 结构。[Ag(Vpe)+][NO3-]-3H2O[分别为 620(2) mg/g 和 137.1(6) mg/g]以及[Ag(Vpe)+][BF4-][分别为 661.8(3) mg/g 和 190(3) mg/g]通过阴离子交换完全吸附了等摩尔溶液中的磷酸根离子和铬酸根离子。DFT 计算表明,在银基配位聚合物中用 Vpe 取代联吡时,扭转能在形成热力学中发挥了重要作用,能耗降低了 4.8 kJ/mol。使用扁平 Vpe 配体获得的结果凸显了配位聚合物在实际阴离子交换中的潜在作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


