Uranyl Naphthylsalophen and Pyrasal Complexes: Oxo Ligands Acting as Hydrogen Bond Acceptors in the Solid State
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Uranium is most stable when it is exposed to oxygen or water in its +6 oxidation state as the uranyl (UO22+) ion. This ion is subsequently particularly stable and very resistant to functionalization due to the inverse trans effect. Uranyl oxo ligands are typically not considered good hydrogen bond acceptors due to their weak Lewis basicity; however, the ligands bound in the equatorial plane greatly affect the strength of the oxo ligands’ hydrogen bonding. In this work, new naphthylsalophen and pyrasal complexes of uranium were synthesized and crystallized for characterization in the solid state. The bond lengths and angles of the uranyl ion and the ligand conformation are compared. In the solid state, one of the pyrasal complexes showed a hydrogen bond directly from a water molecule to the uranyl oxo ligand, which resulted in an asymmetric lengthening of the U–Oyl bonds from 1.789 to 1.862 Å and 1.784 to 1.844 Å.
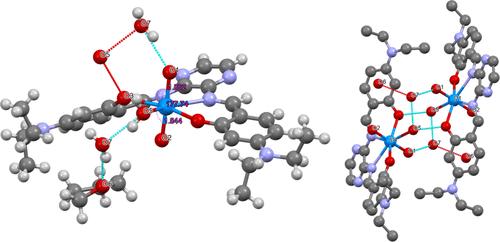
铀酰萘基salophen和Pyrasal配合物:氧配体在固态中作为氢键受体
当铀以+6氧化态暴露在氧气或水中时,它是最稳定的铀酰(UO22+)离子。由于反式效应,该离子随后特别稳定,并且非常耐官能化。铀酰氧配体由于其弱路易斯碱度,通常不被认为是良好的氢键受体;然而,在赤道平面上结合的配体对氧配体的氢键强度影响很大。本文合成了新的铀的萘基salophen和pyrasal配合物,并在固态下结晶进行了表征。比较了铀酰离子和配体构象的键长和键角。在固体状态下,其中一个pyrasal配合物的氢键直接从水分子连接到铀酰氧配体,导致U-Oyl键从1.789延长到1.862 Å和1.784延长到1.844 Å。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


