Cage-Based Metal–Organic Framework Featuring a Double-Yolk Core–Shell U6L3@U18L14 Structure for Iodine Capture
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
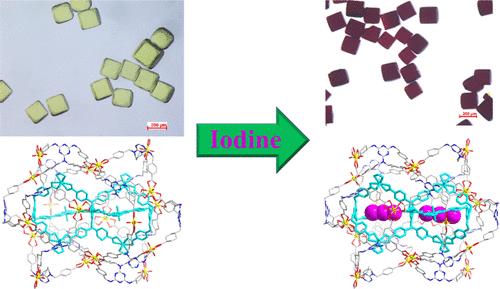
Cage-based MOFs, with their customizable chemical environments and precisely controllable nanospaces, show great potential for the selective adsorption of guest molecules with specific structures. In this work, we have constructed a novel cage-based MOF [(CH3)2NH2]2[(UO2)2(TMTTA)]·11.5DMF·2H2O (IHEP-51), utilizing a triazine derivative poly(carboxylic acid), 4,4′,4″-(((1,3,5-triazine-2,4,6-triyl)tris(((4-carboxycyclohexyl)methyl)azanediyl))tris(methylene))tribenzoic acid (H6TMTTA), as an organic ligand and uranyl as a metal node. The 2-fold interpenetrated (3,6,6)-connected framework of IHEP-51 features two types of supramolecular cage structures: the Pyrgos[2]cage U6L3 and the huge cage U18L14. They are further assembled into a double-yolk core–shell U6L3@U18L14 structure, making it suitable for I2 capture. The maximum adsorption capacities of IHEP-51 for iodine in solution and gaseous iodine are 420.4 and 1561.2 mg·g–1, respectively. XPS, Raman spectra, single-crystal X-ray diffraction, and DFT calculations reveal that the adsorbed iodine is located inside the U6L3 Pyrgos[2]cage in the form of I3–, thus resulting in the formation of a (I3)2@U6L3@U18L14 ternary core–shell structure.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


