Bicarbonate ions promote rapid degradation of pollutants in Co(II)Fe(II)/peroxyacetic acid systems
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Peroxyacetic acid (PAA), as an oxidizing agent, has gained significant attention in the field of advanced oxidation because of its low toxicity and high degradation capacity. In this study, cobalt-iron-based Prussian blue analogs (Co-PBAs) were utilized for the first time to activate PAA for tetracycline degradation. In the Co-PBAs/PAA system, organic radicals (RO•) and high-valent metal oxides are mainly produced. TC is efficiently removed in a wide pH range (5−9) and a variety of interferences (Cl-, SO42-, bicarbonate ions (HCO3-), humic acid, and the actual water bodies) in water bodies due to the specificity of RO•. Interestingly, the catalytic rate of the Co-PBAs/PAA system was significantly accelerated in the presence of HCO3- (kobs increasing from 0.171 min−1 to 0.534 min−1). This enhancement is attributed to the reaction between HCO3- and PAA, and carbonate radicals (•CO3-) and acetyl peroxyl radicals (CH3C(O)OO•) are generated and then react with the phenolic hydroxyl group of TC. In this study, the mechanism of PAA activation by Co-PBAs was revealed, and PAA-based advanced oxidation process enhanced by HCO3- was provided for the removal of pollutants from wastewater.
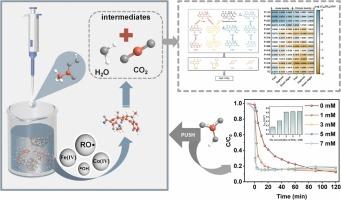
碳酸氢盐离子促进Co(II)Fe(II)/过氧乙酸体系中污染物的快速降解
过氧乙酸(PAA)作为一种低毒性、高降解能力的氧化剂,在深度氧化领域受到了广泛的关注。本研究首次利用钴铁基普鲁士蓝类似物(Co-PBAs)激活PAA降解四环素。在Co-PBAs/PAA体系中,主要产生有机自由基(RO•)和高价金属氧化物。由于RO•的特异性,在较宽的pH范围(5−9)和水体中的各种干扰(Cl-、SO42-、碳酸氢盐离子(HCO3-)、腐植酸和实际水体)中都能有效去除TC。有趣的是,在HCO3-的存在下,Co-PBAs/PAA体系的催化速率显著加快(kobs从0.171 min−1增加到0.534 min−1)。这种增强是由于HCO3-与PAA反应,生成碳酸盐自由基(•CO3-)和乙酰过氧自由基(CH3C(O)OO•),并与TC的酚羟基发生反应。本研究揭示了Co-PBAs活化PAA的机理,提出了以HCO3-为强化剂的PAA高级氧化工艺用于废水中污染物的去除。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


