Flotation enhancement of spodumene with a β-amino-hydroxamate surfactants
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In view of the combination of dodecylamine and hydroxamate can improve the flotation recovery of spodumene, a novel β-amino-hydroxamate surfactant, i.e., N-(3-(dodecylamino)) hydroxamic acid (DAHA), was designed and introduced into spodumene flotation. The micro-flotation performance indicated that DAHA performed excellent collecting ability toward spodumene, and 2.0 × 10-4 mol/L of DAHA floated out about 90.2% of spodumene, while that of sodium oleate (NaOL) was only about 57.4%. The zeta potential of DAHA-treated spodumene revealed the effective chemisorption of hydroxamate group on spodumene surface. FTIR and XPS recommended that the hydroxamate group in DAHA chelated with surface Al sites to form five-membered hydroxamate-(O,O)-Al complexes. The affinity of DAHA on spodumene spontaneously enhanced by the protonated amino group (–NH3+), which facilitated the chelation of hydroxamate with the surface Al atoms and strengthened the hydrophobicity of spodumene.

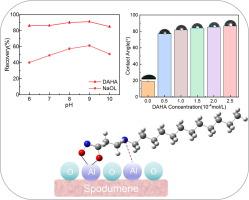
β-氨基羟酸酯表面活性剂对锂辉石浮选的增强作用
鉴于十二胺与羟肟酸盐复合可提高锂辉石的浮选回收率,设计了一种新型β-氨基-羟肟酸表面活性剂N-(3-(十二胺))(DAHA),并将其引入锂辉石浮选中。微浮选性能表明,DAHA对锂辉石具有良好的捕收能力,2.0 × 10-4 mol/L的DAHA对锂辉石的捕收率约为90.2% %,而油酸钠(NaOL)的捕收率仅为57.4% %。经daha处理的锂辉石的zeta电位揭示了羟基在锂辉石表面的有效化学吸附。FTIR和XPS表明,DAHA中的羟基与表面Al位点螯合形成五元羟基-(O,O)-Al配合物。DAHA对锂辉石的亲和作用被质子化氨基(-NH3 +)自发增强,促进了羟酸盐与表面Al原子的螯合,增强了锂辉石的疏水性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
文献相关原料
公司名称
产品信息
阿拉丁
Sodium oleate (NaOL)
阿拉丁
potassium chloride (KCl)
阿拉丁
aluminium chloride (AlCl3)
阿拉丁
methyl isobutyl carbinol (MIBC)
阿拉丁
sodium hydroxide (NaOH)
阿拉丁
hydrochloric acid (HCl)
阿拉丁
Sodium oleate (NaOL)
阿拉丁
potassium chloride (KCl)
阿拉丁
aluminium chloride (AlCl3)
阿拉丁
methyl isobutyl carbinol (MIBC)
阿拉丁
sodium hydroxide (NaOH)
阿拉丁
hydrochloric acid (HCl)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


