Probing Methylmercury Photodegradation by Different Fractions of Natural Organic Matter in Water: Degradation Kinetics and Mercury Isotope Fractionation Characteristics
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
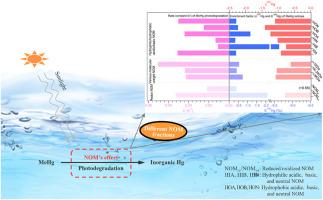
Recent advancements in mercury (Hg) isotopic fractionation research have evolved from conceptual demonstrations to practical applications. However, few studies have focused on revealing fractionation fingerprinting for aqueous methylmercury (MeHg) photodegradation due to its sensitivity to natural organic matter (NOM). Here, the impact of NOM fractions with varying chemical properties on MeHg photodegradation kinetics and Hg isotope fractionation characteristics was investigated. Findings reveal that reduced NOM, containing alcohol/phenol groups, slows the degradation rate compared to the oxidized. Low-molecular-weight NOM, rich in thiol groups, enhances the degradation rate more effectively than high-molecular-weight counterparts. Hydrophilic/hydrophobic-acidic/basic NOM also significantly influence the rate constant, with the highest for hydrophilic-acidic NOM. Isotopic analysis showed that NOM's redox properties affect the extent and direction of Hg isotope fractionation. NOM with various molecular weights controls mass-dependent and mass-independent fractionation by regulating MeHg-NOM triplet radical pairs reactions, likely due to differences in functional groups. Similar effects were observed for different hydrophilic/hydrophobic-acidic/basic fractions. Further experiments with scavenger addition indicated that direct photodegradation of MeHg-NOM is a possible degradation mechanism, with free radicals/reactive oxygen species playing a minor role. These findings underscore the sensitivity of both the degradation rates and Hg isotope fingerprinting to different NOM fractions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


