Synergistic pyrolysis of Cellulose/Fe-MOF Composite: A Combined experimental and DFT study on dye removal
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
We propose the development of an innovative composite material formed through the pyrolysis of oxidized cellulose derived from sawdust, utilizing iron-based MOF as a precursor. This novel material incorporates multiple iron-based components (Fe3O4, Fe3C and Fe0) within a biochar matrix. We employed the composite to adsorb a cationic dye from aqueous solution. Batch adsorption studies explored the effects of pH, contact time, and initial dye concentration. The experimental data fitted well with the pseudo-second-order kinetic model, suggesting chemisorption as the primary mechanism, while equilibrium adsorption results fitted to the Langmuir isotherm model, described monolayer adsorption displaying the highest adsorption capacity (106 mg/g). A fixed-bed column experiment further demonstrated effective removal of methylene blue (MB) dye, achieving an initial breakthrough time of approximately 12 h, and exhibiting an adsorption capacity (qe = 71.14 mg/g) surpassing batch adsorption capacity at the same concentration (qe batch = 52.53 mg/g), signifying the practical utility of the materials. In addition, pyrolysis-derived biochar samples displayed improved total organic carbon (TOC) removal efficiency, with P-Cell-MOF achieving 93 % TOC removal. Density functional theory (DFT) calculations were employed to investigate the binding of MB on the various materials derived from the pyrolysis of cellulose with MOF. The calculations show that MB chemisorbs on both Fe (110) and Fe3C (001) surfaces while only physisorption was observed on Fe3O4(111) and graphene. These computational findings align well with the experimental data and provide an explanation for the enhanced TOC removal observed with the P-Cell-MOF.
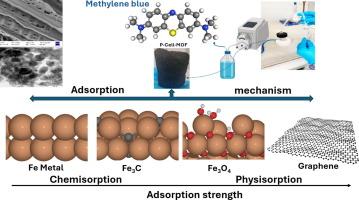
纤维素/Fe-MOF复合材料协同热解脱除染料的实验与DFT联合研究
我们建议开发一种创新的复合材料,通过热解从木屑中提取的氧化纤维素,利用铁基MOF作为前驱体。这种新型材料在生物炭基质中结合了多种铁基成分(Fe3O4, Fe3C和Fe0)。我们利用该复合材料从水溶液中吸附阳离子染料。批量吸附研究探讨了pH值、接触时间和初始染料浓度的影响。实验数据符合拟二级动力学模型,表明化学吸附是主要的吸附机理,而平衡吸附结果符合Langmuir等温线模型,单层吸附表现出最高的吸附容量(106 mg/g)。固定床柱实验进一步证明了该材料对亚甲基蓝(MB)染料的有效去除,初始突破时间约为12 h,吸附量(qe = 71.14 mg/g)超过了相同浓度下的批处理吸附量(qe batch = 52.53 mg/g),表明该材料具有实用价值。此外,热解衍生的生物炭样品显示出更高的总有机碳(TOC)去除效率,p - cells - mof达到93% %的TOC去除率。采用密度泛函理论(DFT)计算研究了MOF热解纤维素得到的各种物质与MB的结合。计算结果表明,MB在Fe(110)和Fe3C(001)表面都有化学吸附,而在Fe3O4(111)和石墨烯表面只观察到物理吸附。这些计算结果与实验数据很好地吻合,并为P-Cell-MOF观察到的增强TOC去除提供了解释。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


