MnO2-based bioresponsive nanoplatform synergizing mitochondrial metabolism modulation for amplified phototherapy and chemodynamic therapy of melanoma
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Phototherapy (PT) against melanoma continues to face significant great challenges with suitable photosensitizers, a hypoxic tumor microenvironment (TME), and aberrant tumor metabolic activities. Herein, we developed a MnO2-based bioresponsive nanoplatform (PIM NPs) that exerted multi-enzyme activities and synergized mitochondrial metabolism modulation for amplified PT and chemodynamic therapy (CDT) of melanoma. The PIM NPs were constructed by coating the MnO2 nanozymes shell onto mesoporous polydopamine nanoparticles (mPDA NPs) loaded with photosensitizer (ICG). The PIM NPs consumed H2O2 to generate O2 under acid TME, alleviating hypoxia to promote the photodynamic effect of ICG for producing toxic singlet oxygen (1O2). Meanwhile, the PIM NPs depleted glutathione (GSH) and triggered the Fenton-like reaction to destroy the antioxidant defense of the tumor cells. The photothermal property of mPDA NPs further enhanced the multi-enzyme activity and the effect of PT. Finally, the bioresponsive PIM NPs down-regulated glycolysis metabolism and oxidative phosphorylation, which disrupted the energy production and nutrient supply of tumor cells, causing the metabolic disorders of tumor cells. Both in vitro and in vivo results showed significant tumor inhibition, indicating that the PIM NPs achieved “All In One” strategy that combining tumor cell mitochondria metabolic regulation to amplify the effect of PT and CDT for melanoma, providing a promising integrated strategy against metabolic abnormalities of melanoma.
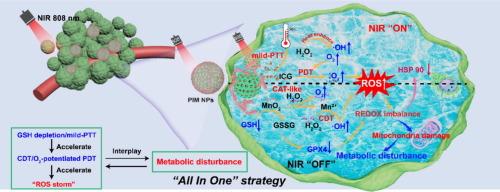
基于二氧化锰的生物反应纳米平台协同线粒体代谢调节用于黑色素瘤的放大光疗和化学动力学治疗
在合适的光敏剂、缺氧的肿瘤微环境(TME)和异常的肿瘤代谢活动方面,黑色素瘤的光疗(PT)仍然面临着巨大的挑战。在此,我们开发了一种基于二氧化锰的生物反应纳米平台(PIM NPs),该平台可发挥多酶活性和协同线粒体代谢调节作用,用于黑色素瘤的PT和化疗(CDT)放大。将MnO2纳米酶包覆在负载有光敏剂(ICG)的介孔聚多巴胺纳米粒子(mPDA NPs)上,构建PIM NPs。PIM NPs在酸性TME下消耗H2O2生成O2,缓解缺氧,促进ICG产生有毒单线态氧(1O2)的光动力学效应。同时,PIM NPs耗竭谷胱甘肽(GSH),触发芬顿样反应,破坏肿瘤细胞的抗氧化防御。mPDA NPs的光热特性进一步增强了多酶活性和PT的作用。最后,生物反应性PIM NPs下调糖酵解代谢和氧化磷酸化,从而破坏肿瘤细胞的能量产生和营养供应,导致肿瘤细胞代谢紊乱。体外和体内实验结果均显示出明显的肿瘤抑制作用,表明PIM NPs实现了结合肿瘤细胞线粒体代谢调节,放大PT和CDT对黑色素瘤作用的“All in One”策略,为黑色素瘤代谢异常提供了一种有前景的综合策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


